|
Diseases
|
BEAK OVERGROWTH AND DEFORMITIES
IN BUDGERIGARS AND PARROTS
Note for Pet Owners:
If your budgerigar or parrot develops overgrowth or deformity of the beak
take it to your veterinarian because there may be an underlying disease
present which needs to be diagnosed and treated with prescription medicines.
|
Description
The beak is usually kept in shape by
it's daily use in feeding and in assisting movement around the birds
environment. Overgrowth of the beak (usually the upper beak, but sometimes the
lower beak) and deformity of the beak are common disorders in pet Budgerigars
and Parrots
Cause
There are several possible causes :
- Inadequate activity, so inadequate
wear
- Malocclusion (the upper and lower
beaks do not meet properly)
- Infection with the mite
cnemidocoptes pilae (budgerigars and cockatiels)
- Inadequate nutrition (eg vitamin A or
D deficiency)
- A local cancer
- Liver disease
- Metabolic bone disease
- Psittacine beak and feather disease
syndrome or "beak rot" (cockatoos and other psittacines)
Breed Occurrence
All Budgerigars and psittacine birds can be affected
Signs
Obvious visible abnormalities of the beak or cere.
Complications
Abnormal beak conformations including beak
overgrowth can lead to difficulty eating, resulting in malnutrition or
starvation
Diagnosis
Examination of scrapings to identify mites, cytology or biopsy to confirm the
presence of a cancer, or other blood tests to confirm the presence of liver
disease.
Treatment
Whatever the cause of overgrowth or deformity the beak should be trimmed
regularly using small scissors and it can be filed down using a small nail file,
or sandpaper. This is to ensure that the bird can continue to eat normally.
Care is needed to avoid over-cutting as this will cause
bleeding. This can be stopped using a silver nitrate pencil - but often the bird
rubs it and bleeding starts again. For this reason beak trimming is best
performed by a veterinarian.
Moving the bird into a larger cage may help increase
exercise and so increase natural wear of the beak. Contrary to popular belief
cuttle bone and mineral blocks are not thought to help with beak growth.
The underlying cause (eg mites) should be treated
appropriately. See cnemidocoptes pilae.
Prognosis
The prognosis is good for mite treatment, but is poor for cancers. Once beak
deformity occurs regular management is usually needed.
Long term problems
Neglected cases may die from starvation and
general debilitation.
Causes of Death of Exhibition
Budgerigars
Dr John R. Baker
The
Liverpool University Budgerigar Ailment Research
Project, sponsored by the Lancashire, Cheshire and North Wales
including the Isle of Man Budgerigar Society, was started in 1984 and ran for
8 years.
In 1988-9 it was decided to find out
exactly what the causes of death of exhibition budgerigars are. All the birds
that died in the large studs of 3 fanciers in the year were post-mortemed. While
in some ways 2 of the studs were not typical, at least two of the findings were
of general interest. First in all three studs the average age of death was
around 2 years whereas the average age of death of pet birds submitted for
post-mortem examination is just over 6 years.
Why should this be so? It seemed
possible that exhibition birds were either more stressed with showing and
breeding than pets or that in breeding for exhibition features, stress
susceptibility had been bred into the birds. Microscopical examination of the
adrenals, the stress glands, did show marked differences between the pet and
exhibition bird but that raised the question as to which was normal, or if
either of them were.
After a year's work dealing with
Australian bureaucracy and getting a file 2½ thick of forms and correspondence,
I eventually managed to get adrenal glands from some wild budgies and it was a
surprise at first to find that they were similar to those of the exhibition
birds. Should this really have been a surprise? In retrospect I think not; life
expectation in the wild is probably less than 1 year and the birds are probably
stressed due to predators, and possible competition, in years when numbers are
high, for food and nest holes. The second thing to come out of this
cause-of-death survey was the importance of quarantine for new birds arriving at
a stud. In two of the three studs there were serious disease outbreaks following
the introduction of new birds which were mixed immediately with the others in
the flight. New birds should be quarantined well away from the resident birds
for at least three weeks. During this time disease may develop, the birds can be
tested to see if they are carrying disease or they can be given preventative
treatment.
Original text Copyright © 1989, Dr John
R Baker.
Clagged Vents
Dr John R. Baker
The
Liverpool University Budgerigar Ailment Research
Project, sponsored by the Lancashire, Cheshire and North Wales
including the Isle of Man Budgerigar Society, was started in 1984 and ran for
8 years.
In 1990-1 it was decided that we would
investigate the condition of clagged vents in which large amounts of droppings
accumulate around the vent and eventually block it and the bird then dies of an
accumulation of waste products in the body. Unfortunately on this occasion the
fancy let the research project down and the number of samples provided meant
that only provisional results could be obtained.
While several causes of the condition
were found it was predominantly due either to kidney disease or to
malfunctioning of the lower part of the bowel. As yet nothing can be done for
the former, which frequently rights itself if the vent is kept clear of
obstructions. In some circumstances the latter is treatable.
Original text Copyright © 1991, Dr John
R Baker.
Diarrhoea in Budgerigars
Dr John R. Baker
The
Liverpool University Budgerigar Ailment Research
Project, sponsored by the Lancashire, Cheshire and North Wales
including the Isle of Man Budgerigar Society, was started in 1984 and ran for
8 years.
After this success with a disease at the
top end of the digestive system (vomiting
budgies), in 1986-7 attention was turned to the other end to look at
why some birds developed diarrhoea. In some ways this was a less successful
investigation, in as much as it did not come up with as neat an answer as the
first two. Over 20 different causes of diarrhoea were discovered but none was
much more common than the others. Some of the causes were within the digestive
system itself; some outside it; some of the conditions were
treatable and others were not.
One of the commoner causes of the
disease was loss of the bacteria which should be present in the intestine. At
the end of the work, all we could do was suggest to the fancy that in cases of
diarrhoea if simple remedies such as cold tea do not work and the problem
persists, laboratory investigation of both the bird and samples of droppings is
essential to find the cause. In many cases antibiotics are not the answer to the
problem. If they are used, and in cases where the gut flora has been lost,
probiotics have a very useful role to play in restoring the germs which should
be present.
Original text copyright © 1987, Dr John
R Baker
DISEASES TRANSMITTED TO
EGGS
by Margaret A. Wissman, DVM, DABVP, Avian Practice
Early embryonic death, blood-ring, dead-in-shell.., these
terms frustrate and confound aviculturists, novice and professional alike. The
reasons that embryos die are many, and diagnosing the specific cause of death
can prove elusive in some cases. The serious aviculturist, whether a hobbyist or
one who makes her living from bird breeding, should work with an experienced
avian veterinarian who can help with all facets of aviculture. Every egg that
dies prior to hatching should be examined by an avian veterinarian who can
perform an egg necropsy and any other tests to determine the cause of death.
Often, histopathology (microscopic examination of tissues)
will prove diagnostic, if the embryo has recently died. Bacterial and fungal
cultures, stains of egg membranes, viral isolation and DNA PCR probes for
specific organisms may also help in diagnosing the cause of death. There are
many infectious organisms that can be transferred from the hen to the egg that
may cause the egg to die. In some cases, the infectious organism may infect the
egg, yet the embryo may continue developing, and may even hatch, carrying the
organism at hatch time. If an organism is passed from an infected hen directly
into an egg, and then into the developing embryo, this is called vertical
transmission. The term vertical transmission also describes the transmission of
an infectious agent from a parent to an egg during fertilization, during egg
development in the oviduct of the hen, or immediately after oviposition. Once
the egg is laid, some infectious organisms can pass through the eggshell upon
contact with contaminated feces, urates or bedding. This is also considered
vertical transmission if infection occurs immediately after laying. Some
organisms are transmitted from the ovary to the egg, and this is called
transovarian transmission. Infectious organisms harbored in the oviduct can also
be passed into the egg prior to the shell being formed. Some organisms infect
eggs if contents from the cloaca contaminate the surface of the egg, and then
penetrate the egg. The other method of transmitting infectious organisms is by
horizontal transmission. Horizontal transmission can occur by preening,
inhalation, copulation, insect or animal bites, ingestion, contact with
contaminated equipment or fighting. It seems obvious that prior to the egg
membranes and shell being applied to it, the egg would be susceptible to
infection by numerous infectious organisms. Even though the eggshell appears
solid, it contains microscopic pores that can allow liquids and small organisms
into the egg. The pores allow the transfer of gasses, as well.
Bacterial Diseases
Chlamydia psittaci is a primitive bacteria that can be vertically transmitted
from an infected hen through the egg to the embryo. Depending on the
pathogenicity of the strain and the number of organisms that are passed into the
egg, the embryo may die during incubation, or it may actually hatch as a baby
bird with chlamydiosis. It should be noted that transovarian transmission of
chlamydiosis has not yet been confirmed by researchers, so it may be that the
eggs are contaminated with the organism by some other vertical method. One
avicultural client of mine with over 100 pairs of large psittacines was having a
problem with a pair of blue-and-gold macaws. They pulled all of the pair's eggs
for artificial incubation. Several eggs in the incubator died about halfway
through incubation. During the egg necropsies, I tested for chlamydiosis by
sending in a swab for DNA PCR testing. I also tested the adult breeder pair for
Chlamydia, using the University of Georgia tests, which included DNA PCR testing
of the blood and DNA PCR testing of a choanal and cloacal swab. A latex
agglutination titer, was run through the University of Miami as well. The eggs
were positive for Chlamydia, as were the parent birds. I recommended that the
breeders remove from the incubator those eggs from the blue & golds, and isolate
them in a small incubator. There were five remaining eggs that were in the early
stages of incubation. To increase hatchability of the potentially infected eggs,
we began a course of egg injections, using injectable doxycycline, which is an
excellent drug for chlamydiosis. The eggs, much to our surprise, continued to
develop, and all five actually hatched on schedule. As soon as the eggs hatched,
I instructed the owners to begin medicating the hatchlings with oral doxycycline,
which would be continued for 45 days total. Because all baby birds receiving
antibiotic therapy should also be prescribed antifungal medication to prevent
infection with Candida sp., we also started the babies on a combination of oral
nystatin suspension and fluconizole.
The babies were also prescribed avian lactobacillus and
acidophilus to give them some normal, good bacterial flora. The five baby blue &
gold’s all developed normally and weaned on schedule. Subsequent testing showed
that these babies showed no signs of chlamydiosis. It should be noted that
testing is not always 100 percent accurate, and although treatment is often
curative, some birds may never completely clear the organism from their system,
resulting in asymptomatic carriers. However, these macaws have thrived and all
have remained healthy. Bacteria of the genus Salmonella can also cause embryos
to die in the shell, or, if the egg is contaminated by a very small number of
bacteria, Salmonella can cause weak-hatch babies that may die shortly after
breaking out of the egg. The bacteria may cause yolk material to coagulate in
the egg, dead embryos may show hemorrhagic streaks on the liver, and the spleen
and kidneys may be congested.
Pinpoint areas of the liver may be necrotic. Inflammation
of the pericardium may also be seen. Salmonella are motile bacteria that can
penetrate the eggshell and are transmitted vertically. Culture of the infected
embryo will prove diagnostic. Some Staphylococcus bacteria can kill embryos. The
avian embryo can be resistant to some strains of staphylococci, but can be
highly susceptible to other strains. Infected wounds on parent birds can infect
eggs, as can staph infections found on the hands of aviculturists, if the egg
comes in contact with lesions. Artificial incubators will grow staph readily,
and it can spread horizontally in this manner. An embryo can die within 48 hours
of exposure to some strains of staph, especially Staph, Aureus. The older the
embryo is at the time of the first exposure to staph, the less chance of
embryonic mortality. Hemorrhages may be found on various internal organs. A
laying hen can develop an ovary infected with Staph, Faecalis, which can
contaminate the forming egg. Contaminated eggs will have up to 50 percent
mortality. Culturing the egg is important for diagnosis. E. coil is a common
bacteria normally found in the gastrointestinal (Gl) tract of mammals, and some
birds as well.
It can enter the egg from an infected reproductive tract
of a hen. E. coil can also penetrate the eggshell if the egg is contaminated
with fecal material. E. coli commonly produces yolk sac infection, causing the
yolk sac contents to appear a watery yellow-green or yellow-brown. Dirty nests
and cages serve as sources of egg contamination. The use of water bottles may
reduce the amount of E. coil that builds up in the Gl tract of birds. In my
experience, aviaries that use a watering system, as opposed to water bowls, have
fewer problems with subclinical bacterial infections in their breeder birds and
their off-spring, Many embryos infected with E. coil die late in incubation or
shortly after hatching. If an E. coil infection is acquired during incubation,
the hatchling may develop an umbilical and yolk sac infection (omphalitis) and
may have poor weight gain. Cracked eggs are more easily infected and may serve
as a source of infection for other eggs in the incubator. Cracked eggs should be
repaired as soon as the damage is discovered, or they should be discarded.
Mycoplasma
Mycoplamatales are one order of microscopic organisms that replicate by binary
fission. They have no cell wall, but have a three-layer membrane. They are more
primitive than bacteria, and must live and grow inside the host. They live only
for a short time. Although we have much to learn about mycoplasmas, they can be
involved in problems with Cockatiel conjunctivitis and respiratory infections,
as well as respiratory/eye problems in other species of pet and breeder birds.
The organism is spread by the respiratory excretions and by the gonads of both
sexes, and infection in the air sacs may lead to contact transmission of the
ovary and developing follicle. Transovarian transmission can occur. Mycoplasma
can spread to the egg from an infected oviduct or from the semen of infected
male birds. It is possible to treat eggs infected with Mycoplasma infections.
Tylosin is injected into the air cell at the start of incubation. A combination
of lincomycin and spectinomycin is also effective for egg injection. Dipping the
eggs in antibiotic solutions reduces the incidence of disease; however, I
personally, have never used this method. A third treatment that has been useful
in breaking the transmission cycle of Mycoplasma gallisepicum and M. synoviae
involves elevating the temperature in a forced-air incubator to 46 degrees
Celsius for 12 to 14 hours before incubating the eggs normally. This technique
inactivates the Mycoplasma organisms, but it will reduce the hatchability by 8
to 12 percent.
Viral Diseases
Several important viral diseases are vertically transmitted in birds. Psittacine
beak and feather disease (PBFD) has been demonstrated to be vertically
transmitted, since the virus is found in the blood of infected birds. It has
been shown that artificially incubated baby birds from PBFD-infected hens will
consistently develop PBFD. So, attempting to control PBFD by pulling eggs for
artificial incubation is futile. Avian paramyxovirus 1 (Newcastle's Disease or
PMV 1) is one of a group of nine distinct serovars (with several more yet to be
characterized) of the virus that is dangerous to birds. Although paramyxovirus
is theoretically vertically transmissible, this mode of transmission is
considered unlikely because infected hens will generally stop laying eggs when
they are viremic. Eggs contaminated by virus-laden feces immediately after
laying can contaminate an incubator, and serve as a source of virus for recently
hatched neonates. Herpesviruses, most of which are quite species-specific,
include Pacheco's disease virus, Amazon tracheitis virus, respiratory disease in
Neophema sp. and Psittacula sp., wart-like or flat plaque-like lesions on the
skin of psittacine birds, budgerigar herpesvirus, pigeon herpesvirus (infectious
to budgies and cockatiels), falcon herpesvirus (infectious to budgies and Amazon
parrots), and Marek's disease (suggestive lesions in budgies). It has been
theorized that some hens latently infected with Pacheco's disease can pass the
virus (and antibodies to the virus) to their eggs. The resulting neonates would
be latently infected carriers that might not develop detectable levels of
antibodies. Herpesvirus of European budgerigars causes feather abnormalities
(referred to as "feather dusters") and is thought to be egg transmitted. It has
been demonstrated in dead-in-shell embryos and is considered a major cause of
early embryonic death in affected flocks, resulting in decreased egg
hatchability. Proventricular Dilatation Disease (PDD) is an enigmatic disease
that is being diagnosed with increased frequency. Although we have much to learn
about this disease, my personal experience indicates that PDD may be vertically
transmitted. I am working with an aviary that has a pair of severe macaws whose
eggs were taken for artificial incubation because the parents often damaged the
eggs after they were laid.
The eggs were placed in a new incubator, and the babies
were the only ones in the nursery during hand-feeding. The owner had problems
with the babies from day one, as the crops were slow to empty, and they did not
gain weight properly. The babies had to be given antibiotics, anti-fungals and
motility enhancers (cisapride) to get them to digest their food at all. One baby
died at six weeks of age, and histopathology showed all the classic PDD lesions.
The second baby died shortly after weaning, and, once again, histopath confirmed
PDD. Histopathological examination of tissues from a dead bird (especially the
proventriculus, ventriculus, crop, small intestines, and brain) is the only way
to confirm PDD in a dead bird as, grossly, many diseases can look like PDD. Dr.
Branson Ritchie at the University of Georgia is currently developing PDD tests,
and when they are available, we hope to test the parent birds of these two
babies. At this time, barium radiographs may render a presumptive diagnosis, and
the biopsy of areas of the gastrointestinal tract may prove diagnostic if
positive. Once the new testing becomes available, it will be easier to screen
for this terrible disease. Some adenoviruses, REO viruses and
reticuloendotheliosis viruses can be vertically transmitted. Influenza A may be
vertically transmitted, as well.
Parasites
Oddly enough, some parasites have been documented to occur within eggs. Adult
ascarids (roundworms) have been found within eggs. These worms get into the egg
by moving from the cloaca up into the oviduct, where the eggshell is then formed
around the aberrant parasite. The fluke, Prosthogonimus ovatus can be found in
the oviduct of Galliformes and Anseriformes, and may also be trapped within an
egg, but the flukes are more likely to result in abnormal eggshell formation.
Conclusions
As our knowledge of avian medicine and theriogenology grows, we may discover
other organisms that can be vertically transmitted. With the information that is
available today, it may be possible to save some eggs that have acquired an
infectious agent. Egg injections are routinely performed in my practice, and
this has greatly increased the hatchability of infected eggs. By testing for
infectious organisms, it is possible to cull eggs, as in the case of PBFD
persistently infected birds, or treat the parents, as in the case of many
bacterial or chlamydial infections. The result is healthier baby birds.
Margaret A. Wissman, DVM, Dip., ABVP-Avian Practise Dr.
Wissman and her husband, Bill Parsons, own and operate Icarus Mobile Veterinary
Service and Small World Zoological Gardens in the Tampa, Florida, area.
Dr. Wissman is a frequent author and lecturer.
French Moult in
Budgerigars - A Review
By: Inte Onsman, Research coordinator
MUTAVI
Research & Advice Group, The
Netherlands
As long as people
breed Budgerigars, French Moult has been the subject of many publications and
discussions. One of the first important publications about this feather disease,
was presented in Diseases of Budgerigars published in august 1951,
Wisconsin USA. At that time French Moult was believed to be caused by faulty
diet, overbreeding, and other factors and even a hereditary form was presumed.
The term "French Moult" was coined in Europe in the last century where the
disease was very prevalent in the 1870's. It had, however been known before that
time. The disease has been called French Moult because budgerigars which were
shipped all over Europe from the large breeding establishments in southern
France, either had the disease in its obvious form or carried it in its hidden
form. These birds when bred, would frequently produce a certain percentage of
youngsters suffering from French Moult. Two manifestations of F.M. were reported
then. The severest form is when young budgerigars in the nest never grow normal
feathers. They have been called runners, creepers, crawlers, or bullets. The
second, milder form of F.M. includes birds which may eventually grow a normal
coat of feathers and become good flyers.
Literature
As early as 1888
Dr. Karl Russ made a summary of statements by breeders on the subject of F.M. as
they had appeared in early bird journals. Dr. Russ, who was familiar with
microscopic work, did not ascribe the cause of F.M. to feather parasites, but to
faulty feeding, overbreeding, and breeding in rooms which are too warm. But the
most comprehensive study on F.M. has been done in 1932 by Dr. Hans Steiner from
the University of Zurich in Switzerland. By continuously inbreeding victims of
F.M. he was able to establish a strain in which this disease was carried on in
the "hereditary" form from generation to generation according to the Mendelian
Law. Dr. Steiner concluded from his experiments that F.M. is the result of
degeneration. The view that mites may cause F.M. has been mentioned many times
during the last 50 years or more, but has been abandoned each time.
In 1950 scientific
work on young Budgerigars suffering from F.M. was done by the Armed Forces
Institute of Pathology, Registry of Vetrinary Pathology, Washington, D.C. The
report published in All-Pets Magazine in May 1950, was very clear. F.M. is not
caused by parasites (including mites) nor by any other "bugs". To find an answer
to the question what really causes this unpredictable disease, more scientific
work was necessary. However, it was not until 1969 that T.G.Taylor published
some experimental investigations on F.M. in Diseases of Cage and Aviary
Birds. He came up with a definition and a describtion of the symptoms and
stated that the latter vary considerably and depend a great deal on the severety
of the attack. He also stated that these variable symptoms have led some people
to suggest that F.M. is not a single disease but that it includes several
distinct, though related, feather diseases. One of the most interesting findings
during these investigations was that a striking difference was found between
bone marrow smears obtained from healthy birds and birds suffering from F.M. Red
blood cells of diseased birds were abnormally fragile and the life span of the
erythrocytes (white blood cells) is unusually short. Taylor concluded, after
testing most theories, that the possibility that F.M. may be due to a virus or a
virus-like agent, should be taken seriously. However, it was not before 1981 the
first serious case report was published in Avian Diseases by Davis and
coworkers [5]. They reported high rates of mortality amongst fledgeling
budgerigars from aviaries in Georgia and Texas. Affected birds died acutely and
exhibited abdomal distention and reddening of the skin. Post mortem they found
enlarged heart and liver with areas of necrosis, and swollen, congested kidneys.
They examined a variety of tissues and found cells with enlarged nuclei
containing inclusions. Electron micrographs revealed the presence of viral
particles in the nuclei in the kidneys, feather follicles, liver, heart, bone
marrow, spleen and brain. Later on in a separate publication also in Avian
Diseases (1981), Bozeman and coworkers isolated a virus that belongs to the
Papovaviridae family [4]. They found characteristics that suggest the virus
belongs to this family, e.g. the presence of DNA, the side of viral replication
appearing to be in the nucleus, and the size of the virus particles ranging from
42 to 49 nm in diameter. (The name Papovavirus was chosen to denote papilloma
(PA), polyoma (PO) and vacuolating (VA) viruses). However,
they referred to the condition of affected birds as budgerigar fledgling disease
(BFD) and characterized a virus (BFDV) isolated from diseased birds.
Again in 1981
another research note was published in Avian Diseases by Bernier, Morin
and Marsolais from Canada [2]. They also reported high mortality rates in
Budgerigars between one and 15 days of age in 19 aviaries in the Province of
Quebec. Signs in adult birds were similar to those described as French Moult.
They considered F.M. to be a milder form of the infection described. Surviving
birds exhibited a retarded growth of flight and tail feathers. Results of their
investigations suggest that this disease of budgerigars is caused by a papova-like
agent that can replicate in many tissues of the body, causing widespread lesions
responsible for the high mortality rate in very young birds.
In 1982 Dykstra
and Bozeman published the results of a light and electron microscopic
examination of a "newly" described avian virus in Budgerigars [8]. They found
that the virus particles are of the same size and symmetry as members of the
polyoma subgroup of the Papovaviridae. The means of transmission of the virus
within an aviary population is less clear, though their studies have implicated
several possible routes. Adults may pass the virus by feeding their young by
regurgitation. Air currents circulating inside affected aviaries may also be
responsible for transmission. They also suggest a possible respiratory route of
transmission since virions within cells of lung tissue were found.
In 1984
Prof.Dr.Kaleta from Germany and coworkers, published some results obtained by
their investigations on young budgerigars showing signs of F.M. [13]. The birds
were purchased from five different sources. Infected cells were isolated and
examined electronmicroscopically. During this investigation intranuclear virus
particles were found with a diameter of 35-45nm.
In the American
Journal of Veterinary Research Dykstra and coworkers published a report to
characterize a virus linked to Budgerigar Fledgling Disease (BFD) which
previously had been identified as a papovavirus [9]. The purpose of this
investigation was to prepare further genetic investigations to develop a
possible vaccine as has been done for e.g. foot-and-mouth disease of cattle.
Their results showed little similarity to simian virus 40 (SV40) or polyomavirus
DNA. During the same year Bernier, Morin and Marsolais published an article in
The Canadian Veterinary Journal involving clinical and pathological
findings in budgerigars suffering from a papovavirus infection [3]. They
described one to 15 day old birds displaying a lack of nestling down feathers
and filoplumes on the head and neck. Microscopic lesions in the feather
follicles of the affected birds less than 15 days of age, were characterized by
focal, multifocal or diffuse ballooning degeneration in the plate cells of the
barb ridges. Again microscopic examination of these cells showed virus particles
similar to those already described by earlier investigators. Results of this
investigation suggested that a papovavirus can cause temporary absence, retarded
growth and the incomplete development of feathers in young budgerigars. The
infection was also suspected to be egg transmitted. The fact that eggs from
pairs producing affected young will also give diseased birds when fostered by
pairs whose own youngsters are normal, supports the hypothesis of
egg-transmission. It was suggested that F.M. is a nonfatal form of the
papovavirus infections described in budgerigars.
Lynch and
coworkers from the Veterinary Laboratory Services Branch, Ontario, Canada, also
published their results in Avian Diseases (1984)[18]. They examined birds
from three unrelated outbreaks of disease occurring in Ontario in 1981,1983, and
1984. Egg-inoculation experiments suggested that the disease may be
egg-transmitted and that significant virus replication must occur before the
budgerigar's immune system matures sufficiently to mount a response.
In the year 1984
many research was carried out on this unpredictable disease. Jacobson and
coworkers reported 45 fledling psittacine birds being raised in an avian nursery
of which 14 died over a 6-week period [12]. Birds died acutely with full crops,
abdominal distention, and hemorrhagic skin. Feather abnormalities were seen in
birds older than 15 days. In this report, a die-off of fledgling conures and
macaws was described. Electron microscopy demonstrated a virus similar in size
and conformation to BFDV (Budgerigar Fledgling Disease Virus).
They concluded that the psittacine papovavirus present in affected birds,
appeared to be related to the polyomavirus subgroup of the papovaviruses. They
also concluded that fledglings from seronegative parents should not be
introduced into a nursery with chicks from seropositive parents. Also in the
same year Pass and Perry from the School of Veterinary Studies, Murdoch
University, Murdoch, Western Australia, described a disease called Psittacine
beak and feather disease [21]. The disease is characterized by loss of feathers,
abnormally shaped feathers and overgrowth and irregularity of the surface of the
beak. The disease was seen in Sulphur-crested Cockatoos, Lovebirds, Budgerigars
and Galahs. During their investigations they found viral particles 17 to 22 nm
in diameter who could be identified in a later state as the picorna virus which
is not a member of the Papova virus family.
In 1985 Pass
published a papova-like virus infection of Lovebirds [22]. The article was
published in the Australian Veterinary Journal and contained information
about similarities to papovavirus infections of psittacine birds described
elswhere.
Krautwald and
Kaleta (1984) investigated 250 budgerigars obtained from 45 different breeders.
Approximately 50 birds came from breeders who never had F.M. in their aviaries
[14]. It was observed that birds who never had any problems with F.M. were very
easy to infect for lack of sufficient antibodies. In the German Cancer Research
Center of the University of Giessen, Lehn and Müller (1986) cloned and
characterized for the first time a virus isolated from fledgling budgerigars,
designated BFDV [16]. A relationship to the polyomavirus subgroup was already
recognized and suggested by previous investigators, but it was now confirmed by
this investigation. Their experiments showed that BFDV is related to but not
identical to the other polyomaviruses. Until now, polyomaviruses had been
isolated only from a variety of mammalian species including man; in contrast,
BFDV represents the first avian member of this subgroup. Lehn and Müller found
strong evidence that BFDV is associated with French Moult.
In 1986 Müller and
Nitschke from the Institute for Virology, University of Giessen, Germany,
investigated a virus isolated from fledgling budgerigars suffering from F.M.
Results obtained by their investigation led to the conclusion that the virus
isolated warrant the classification as a polyoma-like virus [20].
David Graham and
Bruce Calnek reported in Avian Diseases (1987), a papovavirus infection
in 44 parrots of at least 18 species exclusive of the budgerigar [10]. They
compared this infection to a generalized virus infection of young budgerigars
which had been recognized in the United States, Canada, Italy, Hungary, Japan,
and the Federal Republic of Germany. A papovavirus infection was confirmed in 27
of the birds by using antibody tests.
In the Journal
of Veterinary Medicine (1989), Krautwald, Müller and Kaleta from the
Institute of Poultry Diseases and Institute of Virology, University of Giessen,
Germany, examined 298 budgerigars from 49 different flocks in order to obtain
some insight into the aetiology of FM and BFD [15]. The difference between birds
infected with FM and birds infected with BFD is that FM birds mostly survive and
BFD infected birds die when 2-to-3-weeks old. Mortality rates even reached up to
100% in several flocks. However, surviving budgerigars showed disorders of
feathers, similar to those of birds with FM; these birds remained less
developed, and many of them were unable to fly (runners). The structural and
physicochemical properties of viruses isolated from budgerigars with BFD and
from several budgerigars with FM described by Krautwald and coworkers and their
results published in a previous paper (1984) confirm the recently published
classification of BFDV as a member of the polyomavirus group (Lehn and
Müller,1986; Müller and Nitzschke,1986). They proposed to place this virus into
a distinct subgroup within the polyomavirus family.
Regine Stoll and
coworkers (1993) studied molecular and biological characteristics of avian
polyomaviruses from different species of birds including chickens and a parrot
[24]. The chicken polyomavirus is called BFDV-2 and the parrot polyomavirus is
called BFDV-3. The non-mammalian polyomavirus isolated from Budgerigars is now
called BFDV-1 virus. They consider Budgerigar Fledgeling Disease virus (BFDV) to
represent the first avian virus being recognized as a member of the polyomavirus
genus in the family papovaviridae (Müller & Nitschke, 1986;Lehn & Müller,1986).
They also consider French moult of Budgerigars to be a milder and more
protracted form of a BFDV infection resulting in chronic feathering disorders
(Krautwald,1989). It is proposed that the avian polyomaviruses should be placed
in a distinct subgroup within the polyomavirus genus of the family Papovaviridae
and the designation Avipolyomavirus is suggested (Stoll,1993).
The aetiology of
French Moult has been very well investigated throughout the years. It appeared
to be a contageous viral disease caused by a member of the papovaviridae family
and is designated now as the avipolyomavirus. This mammalian virus was obviously
able to adapt, replicate and survive in psittacine birds because of its unique
properties (Griffin,1983) [11]. Two forms of the disease have been recognized in
Budgerigars; the most severe form (BFD) causing mortality rates up to 100% in
budgerigar fledgelings and a mild form called French Moult causing feather
disturbances resulting in birds called "runners". If an outbreak of the disease
occurs, precausions should be taken to prevent the virus from spreading
throughout the aviary (Baker,1990) [1]. Adult birds probably spread the disease
through feather dust and droppings. Runners spread the virus through feather
dander, feather dust and droppings. The infection is egg-transmitted for eggs
from pairs producing affected young will also give diseased birds when fostered
by pairs whose own youngsters are normal. The use of an effective disinfectant
such as Vircon S is recommanded. If the disease is present in your aviary, do
not sell or exhibit birds because if you do so, you will spread the disease to
other fanciers.
Granulocytic Sarcoma in a
Budgerigar (Melopsittacus
undulatus)
AnaPatricia García, Kenneth S. Latimer, W. L. Steffens,
and Branson W. Ritchie
College of Veterinary Medicine, The University of Georgia,
Athens, Georgia 30602.
Abstract. An adult female
Budgerigar was presented with dyspnea and abdominal distention. After a few days
of supportive treatment the bird died and a necropsy was performed. A large,
cystic, perihepatic mass was present. Microscopically, this mass was composed of
multifocal to coalescing aggregates of proliferating heterophils. The neoplastic
cell population showed maturation from the blast stage to differentiated
granulocytes. Electron microscopy demonstrated that the granulocytic cells
belonged to the heterophilic lineage. The final diagnosis was granulocytic
sarcoma.
Key Words: Avian,
Budgerigar, Melopsittacus undulatus, Chloroma, Granulocytic
sarcoma, Heterophil, Myelocytoma, Myeloblastoma
Introduction
Granulocytic leukemia is the neoplastic proliferation of
granulocytes originating in the bone marrow. Infrequently, granulocytic leukemia
in mammals is associated with the formation of sarcomatous tissue masses called
chloromas.1 These neoplastic tissue masses usually arise in visceral
organs. They are characterized by a green color that is imparted by
myeloperoxidase in the specific (azurophilic) granules of neutrophils. Similar
hematopoietic neoplasms in birds are known by the synonyms granulocytic sarcoma,
myelocytoma, or myeloblastoma. These neoplasms are associated with the
proliferation of heterophils and their precursors, which lack myeloperoxidase.
Granulocytic sarcoma is relatively common in domestic fowls but is rare in
exotic birds.1-3 The purpose of this case report is to describe a
granulocytic sarcoma in a Budgerigar.
Case Report
An adult female Budgerigar (Melopsittacus undulatus)
was presented to the University of Georgia Veterinary Medical Teaching Hospital
with abdominal distention and dyspnea. The bird died a few days after admission
despite supportive treatment. Necropsy examination of the animal revealed a
large, cystic, perihepatic mass.
Microscopically, the mass was composed of multifocal to
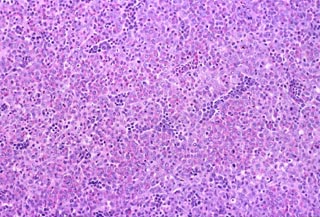
coalescing aggregates of proliferating granulocytes (Fig. 1). This cell
population showed maturation from myeloblasts to more differentiated
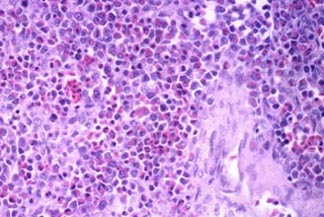
granulocytes (Fig. 2). Cytoplasmic granules appeared red and slightly elongate,
suggesting heterophilc cell lineage. The neoplasm also contained numerous
variably sized and shaped vascular spaces. More normal sections of liver
contained scattered foci of extramedullary heterophil production, often centered
around blood vessels beneath the hepatic capsule.
|
|
|
|
Fig. 1.
Budgerigar, granulocytic sarcoma, H&E stain. Perihepatic mass appears
hypercellular. |
Fig. 2.
Budgerigar, granulocytic sarcoma, H&E stain. More differentiated cells
contain red cytoplasmic granules. |
Electron microscopy of ultrathin sections of the mass
revealed two types of neoplastic cells. The first cell type consisted of large,
round blasts with a thin rim of cytoplasm containing occasional granules. The
second cell type appeared smaller, round , and more differentiated. These cells
had more aggregated chromatin within cell nuclei and more abundant cytoplasm
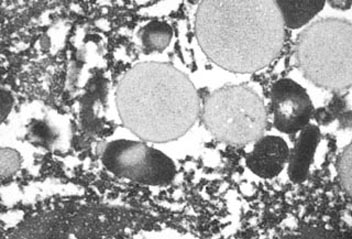
containing membrane-bound granules (Fig. 3). The cytoplasmic granules were round
in cross section and elongated in longitudinal section. Some of the granules
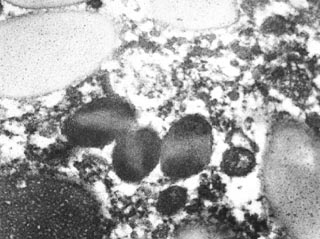
were more electron dense than others and occasionally appeared to have a lighter
central core (Fig. 4). These ultrastructural characteristics were typical of
heterophilic lineage. The definitive diagnosis in this Budgerigar was
perihepatic granulocytic sarcoma.
|
|
|
|
Fig. 3.
Budgerigar, granulocytic sarcoma, electron micrograph. Heterophil
granules are round in cross section and elongate in oblique or
longitudinal section. Notice variation in granule radiodensity. |
Fig. 4.
Budgerigar, granulocytic sarcoma, electron micrograph. More radiodense
heterophil granules occasionally have a lighter central core. Radiolucent
granules also are present. |
Discussion
Neoplasia is a frequent cause of death in pet Budgerigars
(Melopsittacus undulatus), affecting more than 15% of birds that are
examined at necropsy.1 The most common neoplasms in Budgerigars are
carcinomas of the kidney, ovary, and testis. In contrast, hematopoietic
neoplasms are rare in Budgerigars. Carcinomas of the genitourinary tract and
fibrosarcomas in chickens are part of a spectrum of neoplasms that may be caused
by infectious type C retroviruses (avian leukosis/sarcoma viruses). In addition,
two similar forms of hematopoietic neoplasia (designated myelocytomatosis and
myeloblastosis) have been observed in chickens. These neoplastic diseases are
associated with avian myeloblastosis virus and often result in hepatomegaly and
splenomegaly from neoplastic cell infiltration.1 Furthermore,
myelocytomatosis is associated with the formation of neoplasms on the surface of
bones with intimate contact to the periosteum. These neoplasms consist of
compact masses of uniform myelocytes. In contrast, myeloblastosis affects most
parenchymatous organs and is characterized by massive intravascular and
extravascular accumulations of myeloblasts with a variable proportion of more
differentiated myelocytes.2
The avian leukosis/sarcoma viruses are closely related
and, depending on their genetic makeup, cause a variety of neoplasms with short
to long latencies. Some viral strains such as avian myeloblastosis virus, avian
erythroblastosis virus, and the sarcoma viruses contain specific viral oncogenes
that cause rapid neoplastic transformation of target cells with subsequent tumor
development within a few days or weeks. These avian leukosis/sarcoma viruses of
chickens have been divided into six subgroups (A, B, C, D, E and J) on the basis
of their host range in chicken embryo fibroblasts of different genetic types,
interference patterns with members of the same and different viral groups, and
type of viral envelope antigens. Viruses of subgroup A and B occur as common
exogenous viruses in the field. In contrast, viruses of subgroups C and D rarely
have been associated with field disease in chickens. The subgroup E viruses
include the ubiquitous endogenous leukosis viruses of low pathogenicity.2
Subgroup J viruses have been isolated from meat-type chickens and are associated
with an increased incidence of myelocytomatosis.2,3
In chickens and other animals harboring infectious
retroviruses, the virus particles can be detected readily by electron
microscopic examination of normal or neoplastic tissues. However, electron
microscopic search for retroviral particles
in budgerigar neoplasms has been unrewarding.4 Using immunodiagnostic
techniques, avian leukosis viral antigens have been detected in sera from
tumor-bearing Budgerigars using an enzyme-linked immunosorbant (ELISA) assay.5
In addition, renal tumor tissues from Budgerigars were positive for the RAV -2
strain of avian leukosis virus using dot-blot analysis and Southern blot
hybridization. These data suggest that avian retroviruses may be implicated as
etiologic agents of certain neoplasms in Budgerigars. In the Budgerigar of this
report, we did not observed viral particles in granulocytic sarcoma tissue
prepared for electron microscopic examination. Further analysis of neoplastic
tissue will be required to confirm or refute the presence of retroviruses in
this neoplasm.
Granulocytic sarcoma (chloroma, myelocytomatosis,
myeloblastic sarcoma, myelocytic sarcoma) has been reported in four Budgerigars
and a White-tailed Black Cockatoo (Calyptorhynchus funereus latirostris).6
The histologic differential diagnosis for these lesions also includes osseous
metaplasia, extramedullary hematopoiesis, myelolipoma, and hemangiolipoma.7
Osseous metaplasia is recognized by the presence of osteoid and spicules of
mineralized bone associated with the hematopoietic cells. Extramedullary
hematopoiesis occurs with some frequency in birds and may be observed in diverse
locations including the spleen, liver, kidney, adrenal gland, gastrointestinal
tract, heart, and dura mater. Histologically, extramedullary hematopoiesis is
associated with the production of mature leukocytes, erythrocytes, and/or
thrombocytes in any combination. Hematopoietic cell proliferation is
unaccompanied by sarcomatous tissue masses. Myelolipoma consists of the
coproduction of hematopoietic cells and lipocytes. These masses are observed
most commonly in the subcutis and liver, but also may be observed within the
thoracoabdominal cavity.7,8 Hemangiolipoma consists of adipose tissue
and a vasoformative component similar to hemangioma. The diagnosis of
granulocytic sarcoma is based upon the presence of a sarcomatous tissue mass
composed of proliferating myeloblasts that show little differentiation to mature
granulocytes.
Diarrhoea in Budgerigars
An Approach to Treatment
Dr John R Baker
Diarrhoea, otherwise known as
scour, wet vent or, is a common complaint affecting either an individual bird or
the whole stud. Diarrhoea varies widely in appearance from normal colour but
soft or runny, to odd colours, mostly grey or grey-green. If dark, bottle-green,
soft droppings are being produced, this indicates that the bird is not eating
but there is probably nothing wrong with the bird's digestive system. If the
black part off the dropping is normal but the white part is soft or fluid, this
indicates kidney problems which are not covered by this article. As described
elsewhere, the causes of diarrhoea
are many and varied, and some of these are amenable to treatment and others are
not. The aim of this article is to give fanciers some help in the treatment of
affected birds always remembering however, that the best person to advise on
treatment is a veterinary surgeon with an interest in cage bird diseases.
The first thing to do, once you
have spotted that you have a bird with diarrhoea, is to catch it and have a
close look at it to check if there are any other symptoms. Is there any matting
of the feathers on the head indicating that the bird is vomiting as well? Look
at the bird's beak and eyes - do they look normal? If not, it may well not be a
digestive problem. Gently feel the bird behind the keel bone (sometimes known as
the breast bone which can be felt as a hard line along the lower side of the
birds chest) - is it pot-bellied or can you feel a swelling which should not be
there? This could indicate cancer which your veterinary surgeon might consider
attempting to operate on. If you notice these or any other symptoms, consult
your veterinary surgeon as soon as possible. For the purposes of this article we
will assume, apart from the diarrhoea, there is nothing else to be found other
than the bird being fluffed up and perhaps not eating.
Having examined the bird it
should not be put back in the flight where it might spread it's disease to other
birds, but put it in a cage on its own or with other similarly affected birds.
The next thing to do is to decide how the bird is. Is it bright and alert and
looking around at its new surroundings, or is it ill, as indicated by it being
dull, listless, fluffed up and sitting on the floor of the cage?
If the bird is obviously ill,
treatment is urgently required otherwise the bird will die. The first thing it
needs is warmth and it should be put somewhere where the temperature is about
80 Fahrenheit. Ideally, this should be in a proper hospital cage, but the use of
a show cage in the airing cupboard is quite a good idea if you don't have a
proper hospital cage. It will also need fluid; about a teaspoonful (6ml) a day
divided into 5 or 6 doses and also containing a readily digested source of
energy.
If you make up a solution of
2 heaped teaspoonfuls of glucose to 3 pints of water this will supply both
requirements. This should be given warm and directly into the crop with a dosing
tube, (available from good pet shops) which all fanciers should have in stock
and know how to use. If you have not used one before ask a local experienced
fancier or your veterinary surgeon how it is done. If the bird does not respond
to this treatment within a day or two the outlook is not good and it should be
seen by your veterinary surgeon.
If the bird shows little or no
sign of illness, except for the diarrhoea, and is eating and drinking, there is
a possibility that some change in management may have upset its insides. Has it
recently been purchased, been to a show, been introduced to a strange group of
birds, had its diet or water changed, or been given large amounts of green food?
If any of these has happened the chances are that the bird will get over its
problem in a day or two. Most of the birds we receive at the University for
examination have diarrhoea for the first day but nearly always get over it
quickly without treatment. If the problem persists, put the bird back on its old
diet and/or water or take it out of the strange group and see if this does the
trick. Another not uncommon cause of diarrhoea is stress; some birds can not
cope on their own and some can not cope with being mixed with others and, in
both these situations there can be an intestinal upset. If you find, for
example, that each time you put a specific bird in a flight it gets diarrhoea,
this will be one of these stress susceptible birds which are upset by being in
the flight. Remember that the large, bulky, wet droppings produced by breeding
hens are quite normal and should not be confused with diarrhoea.
If there has been no change in
the way you look after the birds and there is a problem with diarrhoea then you
will have to start to think about some form of treatment. The first point to
make about this is that the last thing you should use are antibiotics. Don't
dash for the yellow powder that most fanciers seem to have. The reasons for this
are first, that only very rarely is diarrhoea due to specific disease causing
bacteria which are the only thing which antibiotics will cure and second, the
more you use antibiotics the more likely they are to lose their effectiveness.
Antibiotics do have an important role to play in bird medicine but they are not
the first drug of choice for the treatment of most cases of diarrhoea. The way
to treat the bird is what is termed symptomatically that is, treating the
symptoms rather than a specific disease. What we need is something which will
calm the gut, and two things which are good for this are cold strong tea - about
a teaspoonful - and kaolin (pure kaolin from the chemists, don't use
preparations for treating humans which have other things added) about 1 or
2 drops. These should be given directly into the birds beak or better still with
a dosing tube.
If the diarrhoea persists other
treatments may be tried. The work we have done at the University suggests that
quite a number of cases of diarrhoea are due to a disturbance in the types and
numbers of bacteria in the gut, without a specific disease causing germ being
present. It is necessary in these cases to re-establish the normal germs. There
are two ways of doing this, one way is to collect some normal droppings from
healthy birds, make these into a slurry with a few drops of water and, with a
dosing tube put this into the birds crop. Obviously there is the risk of
spreading disease if this is done and a better approach is to use a probiotic.
These are available from the pet shop under a wide variety of trade names. One
that is normally available is called "Revive" but there are a number of others
which are equally good, on the market. Probiotics are cultures of harmless germs
which are normally present in the birds intestines and the idea is to swamp any
germs which might be causing the problem and re-establish the normal ones and
hopefully, cure the disease. They can also be used to establish germs in the
intestines when they have been lost for some reason. Some of these come with
their own dosing tube holding the right amount and with others the instructions
on the bottle should be followed
If none of these methods work
now is the time to try antibiotics if you have some in stock. If not, contact
your veterinary surgeon and show him the bird and he will probably prescribe
them. If the antibiotics don't cure the problem contact your veterinary surgeon
and ask him to arrange for samples of droppings to be sent off to a laboratory
for examination so that the specific cause of the problem can be identified and
the correct treatment given.
Megabacteria in Diseased and
Healthy Budgerigars
Dr John R Baker
Megabacteria are associated with disease
and death in Budgerigars and a range of other birds. They inhabit the
proventriculus or true stomach where they cause changes in the structure and
function of the organ. The proventriculus becomes dilated and the wall
thickened; the production of digestive juices is impaired; excess mucus is
produced and there may be ulceration at each junction of the proventriculus and
gizzard. The disease is extremely common in exhibition Budgerigars in the UK and
is the major cause of illness and death in these birds.
Megabacteria Carriers
It was believed that apparently healthy
birds could carry this infection and live in balance with it, and that these
birds were responsible for spreading the infection from stud to stud as birds
were bought, sold and exhibited. Some vets, and others, believe that
megabacteria are normal inhabitants of the bird's stomach, and that some other
disease is responsible for the changes in the proventriculus which allows the
number of megabacteria to increase. No proof of this has ever been produced so
work was carried out to establish whether carriers existed or not. Was
megabacteria after all, simply a normal inhabitant of the proventriculus? It was
also hoped that it would be possible to demonstrate the role of megabacteria in
causing proventricular disease.
Diagnostic Focus on Megabacteria
As part of the Budgerigar Society
diagnostic service, dead Budgerigars are examined post-mortem. In 160 birds
received over the last few months, particular attention has been paid to the
proventriculus, and this organ has been examined for megabacteria regardless of
the cause of death. The results were as in the table below.
|
Results of Diagnostic Focus |
|
|
With Megabacteria |
Number of birds |
% of birds in the group |
|
Birds with normal proventriculi |
Yes |
28 |
33 |
|
No |
57 |
67 |
|
Birds with abnormal proventriculi |
Yes |
69 |
92 |
|
No |
6 |
8 |
High
Numbers of Carriers
As only one-third of the birds with
normal proventriculi have megabacteria in the organ, the bacteria cannot be
considered to be a normal inhabitant of this part of the Budgerigar. However, in
most other diseases where clinically normal carriers are present, they form a
lower percentage of the population than this. A possible reason for the high
prevalence of clinically normal carriers, is the very high prevalence of the
clinical disease which results in large numbers of healthy birds being exposed
to the infection and becoming unwell or carriers of the infection.
Other
Causes of Abnormal Proventriculi
The birds with megabacteria infection of
an abnormal proventriculus showed changes, such as an excess of mucus in the
organ or a minor degree of dilation, thickening of the wall, and ulceration at
the junction of the gizzard and proventriculus. Many of these birds had been
clinically ill but, in some of the cases of birds with minor lesions, the birds
had died of other causes. While nearly all cases of abnormal proventriculi were
due to megabacteria infection, a few were not. In this survey six birds had
abnormal proventriculi due to:
- In two cases, E. coli
infection.
- In two cases, ulcers.
- In one case, a cyst.
- In one case, of excess mucus
production of an unknown cause.
Conclusions
For a small survey it can be seen that:
- Proventricular disease is almost
always due to megabacteria infection.
- That there are many clinically normal
carriers in the Budgerigar population which can go either up or down with the
infection when subjected to stress, or remain as carriers posing a risk to
un-infected birds they come into contact with.
- Megabacteria do not appear to be
normal inhabitants of the proventriculus of Budgerigars.
Original text Copyright © 1997, Dr John
R Baker.
Megabacteriosis:
Notes for Budgerigar Fanciers
Dr John R Baker
This is a copy of the notes that Dr
Baker sends out after carrying out post-mortem examinations.
Megabacteriosis is an infection of the
stomach which interferes with the proper digestion of the food, and in the long
term, the affected birds die of starvation. Some of the affected birds show
retching or vomiting and some have diarrhoea. Occasionally birds die rapidly
from this disease, and in these cases there has been the formation of an ulcers
in the stomachs and the birds can bleed to death internally from these.
There appear to be different strains of
this bacteria, some of which cause relatively rapid death, while others cause
mild symptoms which can last for a long periods, months, and occasionally years,
before the condition becomes serious.
Once this infection gets into a stud,
you must expect occasional birds to go down with this disease. This is
particularly prone to occur when the birds are subjected to stress, for example,
when they are put down to breed, taken to shows or moved to a new owner's
premises.
The disease is extremely common and the
majority of studs are affected.
There is a drug which will eliminate the
megabacteria from the birds, but only rather more than half the birds get better
because, in some birds, the damage to the stomach is so severe that it never
heals. To be effective it is essential to treat birds early on in the disease.
It may be worth getting some of the drug to try treatment of any other birds
which go down with weight-loss and the symptoms described above. The drug is
amphotericin B which is sold under the trade name of Fungilin Suspension
(manufactured by E R Squibb and Sons Ltd). Birds should be given 0.1 ml of this
preparation into the beak, or preferably by crop tube, twice a day for at
least 10 days. It is very important that there is no break in the treatment and
that the doses are given at 12-hour intervals. I regret that it is not possible
for me to treat affected birds here. You will be able to obtain the drug through
your local veterinary surgeon; you may have to show him this letter and some of
the birds before he will let you have it.
You may have read in the fancy press
that the condition can be treated with various acids; I have tried a number of
these completely without success
Unless you are prepared to dose all the
birds as detailed above, there is no way of eradicating the infection from a
stud with drugs available here. The Australians do have a drug which can be
given in the drinking water to eliminate this infection, but unfortunately, it
is not available in the UK. The immunity to this infection is weak, so that if
treated birds are exposed to the infection again, for example at a show, or if
an infected bird is bought and added to the stud, they are very likely to catch
it and the disease may reappear.
While I have use amphotericin B in a
very large number of birds without ill effect I am required to tell you that
the product is not licensed for use in birds. This means that if some
adverse reaction occurs you will have no claim on the manufacturers, the
veterinary surgeon prescribing the product, nor myself.
Original text Copyright © 1997, Dr John
R Baker.
Liverpool University
Budgerigar Ailment Research Project
Overview
Dr John R. Baker
The research
project into budgerigar ailments, sponsored by the Lancashire, Cheshire and
North Wales including the Isle of Man Budgerigar Society, was started in 1984
and lasted for 8 years. From 1992 there will be some change of emphasis so this
seems a good time to look back at what has been achieved.
The project starts
each year in February and prior to this discussions are held to decide the topic
for the year. The aim is to pick a topic of interest to the fancy, which stands
a reasonable chance of being completed in the year, and taking into account the
limited time that I can devote to research and the constraints imposed by the
finance available. The latter two are particularly important and mean that a
number of topics of great interest to the fancy, such as French moult, cannot be
investigated.
1984-5:
Going Light
The topic chosen for 1984-5 was 'going
light.' Many diseases of cage birds make them lose weight and 'go light.'
1985-6:
Vomiting Budgies
1985-6 was the year of vomiting budgies.
Birds actually being sick or or trying to vomit were noticed by a number of
fanciers as being a common problem.
1986-7:
Diarrhoea
After this success with a disease at the
top end of the digestive system, in 1986-7 attention was turned to the other end
to look at why some birds developed diarrhoea.
1987-88:
Reproduction
In 1987/88 attention was turned to
reproduction and in particular why exhibition budgies had (and still have) such
an abysmal breeding record when compared with almost all other breeds of cage
birds and poultry.
1988-89:
Causes of Death of Exhibition Budgerigars
In 1988-89 it was decided to find out
exactly what the causes of death of exhibition budgerigars are.
1990-91:
Vitamin and Mineral Supplement Poisoning
I had noticed when talking to fanciers
that many used a great variety of mineral and vitamin supplements and it was not
unusual for 2, 3, or even more of these to be used in one stud.
1990-91:
Clagged Vents
In 1990-1 it was decided that we would
investigate the condition of clagged vents in which large amounts of droppings
accumulate around the vent.
1991-92:
Eye Diseases
The last project, undertaken in 1991-2,
was an investigation of eye problems in budgerigars.
Also in 1990-91 the
University purchased some very expensive equipment for doing blood chemistry on
the normal domestic species that we deal with and this had the capability to do
chemistry on minute amounts of blood. This equipment was used to find out the
normal levels of a number of substances in normal budgerigar blood and,
following this, to use it as a method for locating the problem in sick birds. It
has proved its worth in the diagnosis of liver and kidney diseases and also in
cases of diabetes.
Conclusion
Due to the lack of
support by the fancy for the last 2 projects, and also as we could not think of
projects which would appeal and be practical for 1992-3 at least we were
offering a free treatment and advisory service to members of the LCNWBS; other
fanciers are to use this service but a charge was made for any work done.
While the above
describes briefly some of the "official" projects, a lot of other work has also
been carried out. Quite early on in the project it became clear that there was a
need for a diagnostic service using samples, live birds or post-mortem
examinations and at least as much time has been spent on this as on the
"official" research. Many interesting diseases have been seen and even now new
ones are cropping up. Small projects have been carried out on three of the most
serious diseases; psittacosis, budgerigar fledgling disease and
megabacteriosis.
In brief
psittacosis is treatable and there is certainly no need for fanciers to put down
infected studs. The other two diseases are not currently treatable but we now
know enough about them to advise as to what to do and what to expect.
There have been
other benefits to come out of the project. The first of these is that the
veterinary students at Liverpool University now get some lectures on budgerigar
diseases and a certain amount of hands-on experience. Second we are now able to
offer to veterinary surgeons in practice, an advisory service for budgerigar
problems and this is quite popular. Third, it became obvious very early in
project that fanciers were having difficulty in locating vets with experience of
cage birds and their diseases. As a result of this a list was drawn up of vets
working in this field, and it has proved very popular indeed and has now been
amalgamated with one produced by a commercial company (Vetrepharm) so that
fanciers should always be able to find somebody in their area who can assist
with problems.
Last but not least
there is no point in doing this type of research and clinical work unless the
findings are made available to the fancy and I am most grateful to Cage and
Aviary Birds, The Budgerigar and the News Report of the LCNWBS for publishing
articles based on the work I have done; articles are also published in various
veterinary magazines from time to time to keep the profession aware of what has
been going on.
While the
"official" projects have been stopped, for a year in the first instance, I do
very much hope that the mutual assistance which has been of such benefit to all
parties, the fancy, the research committee, and myself will continue and
flourish for many years to come.
Original text
Copyright © 1992, Dr John R Baker.
Medical Conditions and Diseases of the
Budgerigar
Budgerigar:
Melopsittacus undulatus
Budgies and
cockatiels are the most common pet birds seen in practice. It is important to
understand the most commonly encountered diseases and conditions of these
popular little birds, in order to be able to properly diagnose and treat them.
When dealing with the owners of budgies and cockatiels, do not underestimate
their attachment to these little birds. Offer them the same diagnostics and
level of care that you would the owner of a macaw or Amazon parrot. To many
budgie and 'tiel owners, their birds are true members of the family and they are
willing to have any necessary diagnostics performed, and have any required
treatments administered. Do not compromise the quality of care offered to the
smaller birds.
The budgerigar,
Melopsittacus undulatus, is a fascinating little psittacine with
well-deserved popularity due to its small size and big personality. The budgie
is the best known of all parrots, and has found its way to virtually every
country in the world. It may be considered a domesticated parrot, as it has bred
prolifically in captivity since the mid 1800s. While sexual maturity usually
occurs at approximately six months of age, researchers have shown that young
male budgies may produce spermatozoa within 60 days of leaving the nest. This
rapid sexual development is a physiological adaptation to an arid environment
and enables very young birds to reproduce quickly when conditions are
propitious.
Most parrots are
taken out of the nest as babies to be hand fed to make them more tame, however,
budgies are usually left with the parents until after they are fledged. Baby
budgies tame down very quickly and make devoted, wonderful companions.

Budgie
Statistics
Budgies are about
seven inches in length and weigh between 26-30 grams. English budgies are
slightly larger and weigh proportionally more. The average life span is between
six and ten years, and the maximum recorded life span is 18 years. Budgerigars
are native to Australia, and tend to live in large flocks, although they may
also be found in small parties. In their native habitat, they feed on seeds
procured on or near the ground, and the important food items are seeds of native
grasses. Crop contents studied included grass seeds and a few seeds of
Portulaca oleracea. Budgies have been described as extremely nomadic in
Australia and they can be found inhabiting timber bordering watercourses,
sparsely timbered grasslands, dry scrublands and open plains. It is very
important to note that wild budgies are very active birds, flying great
distances to visit waterholes and seeding grasses. They fly from tree to tree
and scurry through the grass searching for seeds. Many problems with captive
budgies can be directly attributed to the sedentary lifestyle of the pet caged
budgie, when its activity level is compared to that of a wild budgie.
Male budgies can be
excellent mimics and can develop huge vocabularies. Hens may whistle and can
learn a few words, but they are not nearly as loquacious as males. Budgies are
dimorphic. Adult males of most colors, except albino and the very pale pastels,
develop a blue cere. Hens have a lilac or tan cere that turns brownish upon
maturity.

Cockatiel
Statistics
Cockatiels are
usually taken from the nest when they are two to three weeks of age for
hand-feeding. Any of the commercially available hand-feeding formulas are fine
to use. Cockatiels are usually 12.5 inches in length, and weight between 75-125
grams. Larger boned show cockatiels may weigh 10-15 grams more. Feel the
pectoral muscles, if they bulge away from the center keel bone, then the bird is
probably overweight. Sexual maturity may occur as early as six months of age,
and up to 12 months. The recorded maximum life span is 32 years, but on the
average, a cockatiel will live for 15-20 years in captivity, given proper
conditions.
Cockatiels are
dimorphic once they have molted out the baby feathers. This molt usually occurs
at about six months of age. Adult grey males have bright yellow facial feathers
and bright orange cheek patches. Adult grey hens have dull facial feathers. With
the color mutations, adult males have solid primary remiges and retrices. Hens
will have yellow dots on the remiges, stripes of yellow on the retrices. These
marks are subtle. Males are great mimics, and can whistle tunes and talk very
well. Hens will vocalize, and can whistle a bit, but most won't talk.

Nutritional
Disorders
Budgies and
cockatiels consume a primarily seed diet in the wild, and they do seem to thrive
on a seed-based diet. However, pellets, sprouted seeds, fresh fruits,
vegetables, pasta, whole wheat bread and healthy table foods are sound additions
to the budgie diet. A budgie that eats just seeds should have access to a
cuttlebone or mineral block, and should receive supplemental vitamins (but NOT
in the drinking water) as directed by your avian veterinarian. Some budgies and
cockatiels are extremely resistant to dietary changes.
It should be noted
that it can be dangerous to try and convert any bird to a different diet without
first ascertaining that it is healthy. Dietary conversion in a sub-clinically
ill bird can precipitate a health crisis. Always evaluate a bird's health before
to attempting to change a budgie's or cockatiel's diet to make sure that is
healthy enough to withstand the stress of changing the diet.
Budgies, and to a
lesser extent, cockatiels, are very prone to obesity and the problems related to
being overweight. The obese budgie or cockatiel may develop lipomas, which are
benign (non-malignant) fatty tumors. These may be found over the crop area, the
chest or the abdomen, most commonly. In other cases, the bird may develop
generalized lipomatosis, which is a layer of fat over the entire surface of the
body under the skin. Xanthomas, yellow fatty tumors, may also occur. Surgery may
be necessary, especially if the skin over the tumor ulcerates, but often, the
tumor will recur, unless changes are made in the diet and activity level.
Obese birds usually
have some degree of liver problems. When fat is deposited in the liver, normal
liver cells are replaced with fat and over time, if enough normal liver tissue
is destroyed, it can no longer function properly. Birds with fatty liver
syndrome (also called hepatic lipidosis) will suffer from some degree of liver
dysfunction and may bleed excessively, as the liver is responsible for providing
clotting factors in the blood. Hepatic lipidosis is very serious and can be
fatal. Prolonged liver damage may result in the liver becoming fibrotic
eventually, leading to cirrhosis of the liver. Hepatic lipidosis can have
multiple causes, and may have a genetic predisposition. Birds on an all-seed
diet with restricted exercise are prime candidates for hepatic lipidosis.
Thyroid dysfunction may result in obesity. Toxins such as aflatoxins can result
in fatty changes in the liver. Steroid administration (from topical ointments or
hormone injections with methylprogesterone, for example) can cause a bird to
gain excessive weight.
Budgies and
cockatiels with liver disease may have overgrown toenails and beaks. The best
indicator for liver disease in birds is the bile acids blood test. To
definitively diagnose liver disease, a liver biopsy should be performed.
However, in many cases, a bird with advanced liver disease cannot withstand the
stress of a surgical procedure and may have problems with the blood not
clotting, so surgery may not be recommended or possible.
While I do not
condone allowing any pet bird free-flight in the home due to the risk of injury
or escape, it must be remembered that budgies and cockatiels are naturally very
busy birds, so the largest cage that is practical should be purchased. They
should have toys, swings, ladders and playgyms, and owners should encourage
their birds to play and exercise frequently to prevent obesity.
Obese birds, and
those with lipomas, should be offered a diet lower in fat. Each case should be
discussed individually with your avian veterinarian. As guidelines, I usually
recommend decreasing regular seed mix and increasing millet, since millet is
lower in fat than the other seeds. Sprouted seed is healthier, and should be
offered daily. I also recommend offering pasta and whole wheat bread, plenty of
fresh veggies and fruit, and some table foods (without butter, margarine or
other added fats). Increasing the activity level of obese birds should be
undertaken.
It should be noted
that feeding greens, fruit and vegetables does NOT cause diarrhea, as is often
quoted in budgie books. Consuming foods with more water in them will cause
increased urination, and not diarrhea. Feeding vegetables and some fruits is
recommended and bird owners should not be put off by printed misinformation that
discourages them from feeding those healthy foods.
Iodine deficiency
in the diet may result in thyroid dysplasia in budgies, although this is not as
common as it used to be. This occurs in birds that consume an all-seed diet that
is deficient in iodine. Birds with thyroid dysplasia present with respiratory
signs from the enlarged thyroid glands pressing on the trachea and syrinx. Some
develop a characteristic squeak when they breathe. This is treated by
prescribing an iodine supplement. Injectable iodine: 20% sodium iodine in saline
for injection, 0.01 ml/budgie once, IM. Oral: Make stock solution of 2 ml
Lugol's iodine in 30 ml water. Mix one drop of stock solution in 250 ml drinking
water. Use daily for treatment, 2-3 times per week for prevention.
Cockatiels should
probably not receive a 100% pelleted diet. Cockatiels that have been on a
pelleted diet for years have developed renal disease. For this reason, I
recommend not feeding more than 50% pellets, some seed, and a good portion of
the diet should be table foods, fruits, veggies, pasta, whole wheat bread, and
other nutritious items. Avocado, chocolate and onions should not be fed, due to
potential toxicosis problems.

Parasitic
Diseases
Budgies with a
crusty cere, feet and vent are usually infested with the Knemidokoptes mite.
Most budgies with this condition are young (usually less than one year of age).
These mites do not cause pruritis (itchiness), and cause a honeycomb type
appearance to the skin and cere, upon close examination. Scrapings of the
lesions or examination of the crusts in oil under the microscope will show the
mites. The treatment of choice is ivermectin based upon careful dose calculation
Dosage: 0.2 mg/kg PO, repeat in 10-14 day intervals until signs decrease.
Although they do not appear to be very contagious, it is recommended that all
birds kept in the same cage also be treated with ivermectin, either orally or
topically. As with demodectic mange in dogs, this mite appears to be related to
the immune status of the bird, and often the offspring of infested birds will
develop Knemidokoptes, as well. Treatment should be repeated at two-week
intervals until the bird is clinically normal. Long term infestation may result
in permanently deformed beaks, which will require periodic shaping by an avian
vet with a grinding tool and emery board. Mites do not live off the bird, so
treating the cage is not necessary, but is recommended. Mites that occur in
older birds usually indicate some underlying medical problem, such as hepatic
lipidosis, diabetes mellitus or even tumors. Mites occasionally occur in other
species of birds, rarely cockatiels.
Red mites can occur
in budgies and cockatiels. These mites are very contagious between birds of
different species, and they suck blood. They are visible to the naked eye as
tiny specks of red pepper. Red mites (Dermanyssus species) remain off the
bird and climb on the host to take a blood meal. They can make the infested
birds very nervous and irritated. They sometimes bite people when birds are
absent. In addition to treating the birds with red mites, the entire cage and
bird area must be thoroughly disinfected to prevent reinfestation. Treatment
with oral ivermectin and topical 5% carbaryl, repeated weekly, is usually
effective. I saw one case involving a military macaw that had a severe
infestation with red mites, and the poor baby bird had multiple feather cysts
caused by the damage from the mites.
Feather mites can
occur on budgies, and two species have been described to infest budgies. These
mites, however, are not commonly encountered. Feather and quill mites can be
found (rarely) on cockatiel feathers (usually primary and secondary remiges).
Many budgie and 'tiel owners believe that they must use some sort of protection
against mites, which can be hung on the outside of the cage, but these are
ineffective and potentially dangerous, as the fumes can cause liver damage and
perhaps cancer if inhaled for a long period of time. Mite protectors usually
have mothballs (paradichlorobenzene) as the active ingredient. If a budgie does
not have mites, a mite protector is not necessary to prevent infestation. If a
budgie does have an external parasite, it is best to seek the expert advice of
an avian veterinarian who can diagnose the exact problem and prescribe the
correct medication to treat it at the proper intervals.
Giardia and
ascarids (roundworms) frequently occur in budgies and cockatiels. (Don't forget,
I practice in Florida, the land of bugs and parasites!) It is important to
realize that birds need not be kept in walk-in aviaries (with access to the
ground), nor do they have to be housed in sub-standard conditions to harbor
these parasites. I have found that ivermectin is not the most effective dewormer
for ascarids. Pyrantel pamoate is a better choice. Trichomonads occasionally
occur in budgies and cockatiels, as well, and this parasite is most often
diagnosed from a swab taken from the oral cavity or crop.
I recommend that
avian vets routinely deworm a pet bird at least once or twice, during routine
first examination, with a very safe medication, even if fecal parasite
examinations are negative. This is because the gastrointestinal tract transit
time is so fast in birds that worm eggs do not concentrate in the fecals (as
they do in dogs and cats), and it is not only possible, but likely, to miss
roundworms on a fecal exam. I have performed countless second opinions on small
birds with GI problems that have had negative tests for worms, yet, when I
dewormed them, they passed roundworms! If a bird passes roundworms, it should be
periodically dewormed for the rest of its life, as the worms may form cysts in
the tissues that are released from time to time, resulting in additional worms
setting up housekeeping in the intestines.
Dosages:
- Pyrantel pamoate
0.1 ml/kg PO
- For Tapes:
praziquantel 5.7 mg/kg IM
Note:
fenbendazole can cause fatal hepatopathies, and should not be used in pet birds
routinely, also causes feather abnormalities if administered when bird is
molting
Giardia,
a one-celled protozoal intestinal parasite is often difficult to diagnose in
birds. Wet mounts of fresh droppings may sometimes show the protozoa. However,
other tests may be necessary to diagnose Giardia. An ELISA (enzyme-linked
immunoassay) test on the droppings may be performed. One lab has had great
success in diagnosing Giardia (and other parasites) by having the vet
place feces in 5% formalin (not the usual 10% used to preserve tissues for
histopathology) which prevents damage to the fragile Giardia cysts, and
then the droppings are examined with special stains and microscopes.
The lab I recommend
is:
Parasitology Research Lab, LLC
P.O. Box 10/357
Neosho, MO USA 64850-0357
Phone/fax: 417-451-0201
Call to set up an account and get fee schedule.
Treatment of
Giardia can be problematic, and most available drugs only kill Giardia
in a low percentage of birds. Metronidazole works in about 30-40% of cases.
However, it has poor palatability, as well. Ronidazole or dimetronidazole are
more effective. Fenbendazole may work well, however, due to potential hepatic
toxicosis, this should be avoided. One way to prevent reinfestation is by having
a bird use a water bottle in place of the more common water bowl.

Infectious
Disorders
While budgies and
cockatiels are very hardy little birds, bacterial infections are commonly
encountered. Most occur in birds that have a water bowl or cup and not a water
bottle. Even the most diligent owner cannot prevent fecal and food contamination
of water in an open vessel, which will rapidly grow bacteria. Budgies and 'tiels
are very smart little birds and most will rapidly adapt to using a water bottle.
Even though budgies
and 'tiels are very small birds, it is possible to run complete blood counts and
blood chemistry panels on them. The complete blood count is probably the most
important single test that can be run on a bird, as it helps the avian vet
determine if a bird has a bacterial, fungal or viral infection, and it shows if
a bird is anemic, dehydrated or suffering from a myriad of problems. Bacterial
culture of the eye, respiratory tract, choanal slit, crop, cloaca or lesion may
help diagnose bacterial problems. Fungal cultures may be helpful. A Gram's stain
may help screen birds for potential problems, however, it is only useful as a
screening test, and not as a true diagnostic test in most cases. Usually, a
healthy bird will have predominately Gram positive cocci and rods. Gram negative
rods are not normally found in high percentages in most birds. An abnormal
Gram's stain should be followed up by appropriate culturing. Candida may
be picked up on Gram's stain, as well, but some foods add brewer's yeast to the
diet, which may show up on the Gram's stain. If yeast is causing problems, it
may be budding or have pseudohyphae. When in doubt, ask for a fungal culture,
which will differentiate between brewer's yeast and potentially pathogenic
Candida.
Yeast infections
with Candida can occur, especially in birds that don't get enough vitamin
A in the diet. Many bacterial and yeast infections in budgies and cockatiels
occur as a result of owners allowing birds to eat out of their mouths, or if
they allow a bird to have contact with human saliva. Our mouths contain many
microbes that can cause disease in birds, and contact with the human mouth
should be strictly prohibited.
A controversial
disease in budgies is called megabacteria. These large bacteria can be found in
the proventriculus (and sometimes the crop or other portions of the GI tract) of
both healthy and ill birds. It can be difficult to diagnose in live birds. Some
researchers believe that megabacteria are normal inhabitants of the GI tract and
others feel that this organism is responsible for causing disease in budgies.
This can also occur in other birds, including cockatiels.
The bacteria
responsible for causing tuberculosis, Mycobacterium, may cause disease in
budgies and 'tiels. One screening test is called an acid-fast stain, and the
organism may also be grown on culture, but a special lab and special techniques
must be used to culture it. Often, birds with TB will have an elevated white
blood cell count.
A primitive
bacterium is responsible for causing the disease known as chlamydiosis (also
caused psittacosis or parrot fever). This organism may cause respiratory disease
in budgies and cockatiels, and is contagious to other birds through infected
droppings, and infected secretions. It may also affect the liver and GI tract.
Budgies and 'tiels can be carriers of chlamydiosis, and may not show clinical
signs. Chlamydiosis is fairly common in budgies and cockatiels, and it should be
noted that testing is not always accurate in the live bird. DNA PCR testing is
helpful in procuring a diagnosis and the white blood cell count may be mildly
elevated, or it may remain normal. Chlamydia is very host-adapted to
cockatiels and diagnosis is very problematic in the live bird.
Due to potential
legal problems, every cockatiel owner should be advised about the zoonotic
potential of chlamydiosis. I give every 'tiel owner a hand-out (purchased from
the AAV publications office). If an owner declines testing, be sure to note that
in the chart.
AAV Publications
office address:
Kathy Lyon
Publications Coordinator
P.O.Box 210732
Bedford, TX USA 76095
PH: 817-428-7900
e-mail: AAVPubs@aol.com
AAV Central Office:
Adina Rae Freedman, CAE
Executive Director
P.O.Box 811720
Boca Raton, FL USA
PH: 561-393-8901
e-mail: AAVCtrlofc@aol.com
Website: www.aav.org/aav
Another disease
that may present with similar clinical signs is mycoplasmosis. Both chlamydia
and mycoplasma may be treated with doxycycline or enrofloxacin. Enrofloxacin
dosage: 7.5-20 mg/kg q12 hrs. For flock treatment: water-soluble doxycycline is
available from Global Pigeon Supply, phone: 912-356-1320.
Budgies are often
implicated in outbreaks of polyomavirus infection. This virus can cause budgie
fledgling disease. French moult, a slow, debilitating disease of budgies may be
caused by polyomavirus or psittacine beak and feather disease virus (PBFD). A
vaccine against polyomavirus is available to protect non-budgerigar psittacines,
and a vaccine for budgies is under investigation. Cockatiels may also suffer
from polyoma viral infections. Budgies and cockatiels may also be affected by
other types of viruses, including Proventricular Dilatation Disease, PDD.

Reproductive
Problems
Budgies are
determinate layers, meaning that a hen will lay a predetermined number of eggs
per cycle, usually four, six or rarely eight. This is in contrast to cockatiels,
called indeterminate layers. A cockatiel hen will continue to lay eggs to
replace those lost from her clutch. So, if an owner takes the eggs away from a
hen as she lays them, she may lay so many eggs (the record in my practice is
27!) that she becomes depleted of her nutrients, which can be life-threatening.
However, the budgie will usually cease laying once her clutch is complete, even
if the eggs are taken away from her. This is why so many more cockatiels end up
with egg-related problems when compared to budgies.
Hypocalcemia can be
treated with vitamin D3 injection, injectable calcium (Calphosan: calcium
gluconate 50 mg/ml and calcium lactate 50 mg/ml, IM, 5-10 mg/kg q12 hr PRN. Make
sure bird is well-hydrated when giving injectable calcium. I usually dilute
50:50 with sterile saline for IM injections). Neocalglucon in the drinking water
can be administered in the drinking water, 1 ml per ounce (30 ml) drinking
water.
Male budgies of
many colors, excluding some of the pale colors, will develop a blue cere as they
mature. The hen will develop a tan or brown cere upon maturity. Some hens will
develop brown hypertrophy of the cere, a thickening of the cere that is
considered normal, however, for cosmetic reasons, an avian veterinarian can
carefully peel off the excessive growth. If the cere color of a mature male
budgie changes from blue to brown, this is often an indication of a tumor of the
testicle.
Occasionally, a hen
may have difficulties in laying an egg. This is termed dystocia, and it may have
many causes, including not enough calcium in the diet resulting in a
soft-shelled egg, a weak or infected oviduct, malnutrition, a damaged or small
pelvis, a malformed egg, poor muscle tone and body condition of the hen or
tumors. Supplying the hen with heat and humidity may help her pass the egg,
however, dystocia can rapidly become an emergency, requiring immediate
veterinary care. Applying mineral oil or water-soluble jelly to the vent is not
likely to be effective, unless the egg is clearly visible just inside the vent.
Many egg-bound hens are dehydrated, so the first treatment an avian vet will
perform is usually the administration of fluids. Radiographs (x-rays) may be
helpful in confirming dystocia. An injection of calcium may help the hen by
allowing the oviduct to contract more strongly. An injection of vitamin D helps
calcium absorption. If fluids, the vitamin injection and the injectable calcium,
in addition to heat and humidity don't facilitate passage of the egg, a
prostaglandin gel (Prepodil gel) may be applied inside the vent, as long as the
egg can physically pass. The prostaglandin gel works along the natural pathways,
and is a much better drug to use in place of oxytocin, which is the drug that
was classically used to cause contractions of the oviduct (and is used in
mammals). Extreme care must be taken by any female personnel dealing with this
gel, as it can cause serious problems with the female reproductive tract.
Occasionally, a hernia may be associated with egg-binding.

Neoplasia
Budgies often
suffer from a myriad of tumors as they age. Budgies that develop a limp or
weakness of one leg often have a tumor pressing on the sciatic nerve, causing
the leg problem. The tumor may be of the kidney, gonad or adrenal gland. Other
tumors may occur in almost any organ. Cockatiels can also develop malignancies
as they age. Fibromas and fibrosarcomas are the most commonly seen in my
practice.

Endocrine
Disorders
Hypothyroidism,
although not well-described, may occur in budgies. Diabetes mellitus, also quite
rare, occurs in budgies, as well. Tumors of the adrenal or pituitary gland have
occurred. Cockatiels, especially, fat, female and four, may develop diabetes
mellitus. In birds, diabetes is due to an overproduction of glucagon, as opposed
to a lack of insulin in mammals.

Gout
Gout is quite
common in budgies. It may be a result of kidney disease or it may be primary in
origin. If urates are deposited in the joints, this is called articular gout.
This is a very painful condition and treatment is usually unrewarding. Visceral
gout, where urates are deposited on the surface of organs, such as the heart and
liver, is usually diagnosed at necropsy. Gout also occurs in cockatiels, and is
usually secondary to renal disease.

Injuries
Budgies and
cockatiels, being the inquisitive little birds that they are, may get underfoot,
and may be injured by being stepped on, closed in a door, etc. Fractures of the
wing or leg frequently occur, and can be usually simply treated by using a tape
splint. Bird bones heal quite rapidly, so splints won't need to be kept on as
long as one would for a dog or cat.
Budgies and
cockatiels may get a concussion from flying into a window or mirror, or injuries
may occur by an encounter with a ceiling fan. This is clinically called
"shredded tweet."

Regurgitation
A budgie may
regurgitate to a favorite toy, mirror or its favorite person. This is a normal
behavior. This must be differentiated from regurgitation resulting from disease.
Cockatiels may also regurgitate to a favorite person or toy, but not as commonly
as in budgies.

Conclusions
Budgies and
cockatiels are wonderful little birds with great ability to mimic speech. They
can be loving companions. Veterinary care is important for them, and just
because they are small birds, their preventative medical care should not be
neglected. Avian veterinarians are able to provide the same quality of care for
a budgie as for a macaw. Always offer the budgie and 'tiel owner the same
diagnostic plan that you would for the owner of a larger bird. With proper care
and diet, the budgie and cockatiel can live a long, healthy life.
Osteopetrosis
Dr John R Baker
Osteopetrosis is a rare disease
of mammals and birds. The name means "stone bones" suggesting that the bones are
even harder than normal although this is not always the case. While the disease
has been diagnosed in various types of domestic poultry there appear to be no
reports of it in cage birds.
The four budgerigars in which
the disease was seen all belonged to the same fancier, and were from two
clutches which also included some normal chicks. The condition was first noted
when the birds left the nest boxes but may have been present before this.
The affected birds had
difficulty in moving about but this varied in severity from bird to bird; one
could only get about at all by hooking its beak into roughness on the cage
floor, while another stood in a slightly crouching position, but could walk and
fly, at least as far as the nearest perch. The birds had normal appetites and
were in good bodily condition. Once the condition was diagnosed in the most
severely affected bird the others were put down and two of the four bodies were
examined post-mortem.
The post-mortem examination
showed that some of the bones were thickened, especially the tibia (drumstick).
Three of the bones of the wing, (humerus, radius and ulna), the ribs and the
bones of the shoulders, (the wish-bone, shoulder blade and the coracoid). Not
all of these bones were affected in individual birds; the one which could stand
had unaffected leg bones. Diseased bones were swollen along their shafts to two
or three times the normal diameter by a layer of new soft bone laid down on
outside of the original shaft of the bone, which still persisted.
In addition there was new bone
completely filling the marrow cavities of the affected bones. As seen in the
budgerigars the affected bones were soft. In most species the new bone
eventually becomes very hard and stone-like but these birds were not allowed to
live long enough to see if this would happen. The bones not affected by this
disease were a little soft and more easily broken than normal, possibly because
of an insufficient intake of calcium for the formation of both the normal and
the diseased bone. The muscles of the legs were very small although otherwise
normal, and this, possibly coupled with pain, was the explanation for the birds'
inability to stand properly. The small size of the muscles meant that the legs
were not noticeably swollen. The wing muscles were unaffected, possibly because
the underlying breast bone was normal.
In other animals there are two
causes of this disease. In most mammals it is inherited; when two animals which
are unaffected carriers of the disease mate, a proportion of their offspring
will inherit two of the recessive genes and will go down with the disease. In
domestic poultry the disease is caused by a virus which means that the conditon
is infectious; however, it seems very probable that only the young chicks are
susceptible to infection. When the disease in was studied many years ago, in
order to get experimental cases young chicks had to be exposed to the virus at
not more than a few days old but the disease did not develop for a few weeks
after birds were infected.
In the cases reported here, it
is not known if the disease was the inherited form or the infectious form; the
parents could have been carriers of either the defective genes or the virus.
However the chance of mating two sets of different males and females all of
which carried the defective gene seems somewhat remote so that the infectious
theory seems more probable. The fancier concerned also kept poultry and while he
had not recognised the infection in them it is possible that one or more of them
were carriers, and the fancier transferred the infection into the budgerigar
stud; this does, however, remain unproven.
There is no treatment for this
disease.
The recognition of new diseases,
albeit rare ones, is one of the valuable outcomes of the veterinary service
offered to members of the Budgerigar Society, as the more diseases are
recognised the better will be our understanding of the causes of illness in
these birds. While the poultry were not proven to be the the source of the
illness this disease outbreak does suggest that totally dis-similar birds can
have the same diseases - there are other diseases of poultry which can and do
spread to budgerigars.
This research has been entirely
funded by the Budgerigar Society.
Original text Copyright © 1997,
Dr John R Baker
Research on Feather Diseases - Part
I
Dr John R Baker
Less common problems are dealt with
in
Part II.
Over the past few years fanciers have
increasingly reported feather abnormalities in their exhibition type
budgerigars. In particular, they have noticed a tendency for birds to lose their
tail feathers which then fail to regrow ("tail-less wonders"). Reports of
similar abnormalities in larger members of the parrot family increased at the
same time.
In view of these reports, discussions
took place between the Budgerigar Society Lancashire, Cheshire & North Wales BS
and myself. It was decided that the two societies would fund work on feather
diseases to be carried out at the University of Liverpool. Their financial
support is acknowledged with thanks. The work was begun on 1 July 1993 and
concluded on 30 June 1995.
We are grateful to members of the fancy
for the supply of birds and feathers together with related information. Birds
and other samples were received from all areas ranging from the Isle of Wight to
Glasgow and from Cornwall to Norfolk, though the majority came from Lancashire,
Merseyside, Cheshire and North Wales. The total number of birds and feather
samples received was 198.
Results
Published to Veterinary World
A technical version of this report will
be produced and submitted to the veterinary press, making the results available
to veterinary surgeons. They will then know which diseases commonly occur and
will be able to advise clients with feather problems in their birds.
Results
The diseases and other abnormalities
found in the survey are summarized in
Table 1 (at the bottom of this page).
Also in the table are figures from the other comparable work found in the
literature. This was a survey of biopsies (small pieces of tissues taken from
live animals) from a variety of psittacines with feather disease, which were
examined microscopically. The work was carried out in America (Schmidt 1987). It
will be noted from the table that the diseases found in the American survey were
of a limited nature.
Only some of these were seen in my
survey but at a different prevalence. The exception was psittacine beak and
feather disease, where the incidence was equivalent in both surveys. In the
Schmidt survey the causes of the diseases were not investigated, nor was there
any attempt to correlate certain diseases with feather type. Caution is required
when interpreting the figures in Table 1. The figures are correct for the birds
we received, but some fanciers supplied large numbers of birds with the same
condition and this has skewed the results. This was particularly true in the
case of psittacine beak and feather disease, where two breeders supplied over
half the affected budgerigars. With pulpitis, the same tended to occur, though
to a lesser extent. Conversely, many fanciers did not submit birds with French
moult as this disease was specifically excluded from the project. For these
reasons the figures in the table give only a rough guide to the prevalence of
these conditions in the overall budgerigar fancy.
It will be noted from Table 1 that there
was an average of 1.48 conditions per bird. Most of these were cases of mite
infestation and damage in birds with other feather disorders. In other cases two
apparently unrelated feather conditions occurred in individual birds.
Terms
Before considering the results in detail
it may be useful to define a few terms.
Viruses
These are a
type of germ which lives and breeds inside the cells of animals. They are
capable of living outside the animal but will not multiply in this situation. As
far as birds are concerned no treatment for viral diseases is available.
Bacteria
These are
much bigger than viruses and usually live between the cells of the infected
animal and multiply both within the animal and outside. They can usually be
killed by antibiotics.
Congenital Disease-
This is a
disease which the bird has from the time it hatches, although occasionally the
symptoms may not show for months or even years. Such diseases may or may not be
inherited.
Inherited Disease
This is a
disease which is passed from parent to offspring via the animal's genes.
Depending on the mode of the inheritance the parents may, or may not show
evidence of the disease.
Vertical Transmission of a Disease
This is
transmission of a disease from parent to offspring by contact or via the egg.
Such diseases are not inherited although they may appear so.
Horizontal Transmission of a Disease
This is
transmission of a disease to other in-contact birds but excludes parent to
offspring transmission.
Follicle
A small
pocket-like depression of the skin from which the feather grows and by which it
is attached to the bird before a moult.
Barb
The branches
coming from the feather shaft and forming the bulk of the feathers.
Barbules
These branch
off from the barbs and have small hooks on them (barbicels) which lock together
the barbs and thus the feather.
The part of the report which follows
details the findings in each condition or groups of conditions and gives an
indication of the cause and treatment when this is known.
Psittacine Beak and Feather Disease
It came as a considerable surprise that
this topped the list of diseases occurring in 21.2% of all the birds submitted,
because in the first year no cases were seen at all. One possible explanation is
that the disease has only recently been introduced into the exhibition
budgerigar and only became widespread in the last year or so.
The disease is caused by a virus.
Infected birds may be symptomless carriers although no such cases were found in
the survey. This was to be expected as only sick birds were seen. Fanciers were
unlikely to send in a symptomless bird for examination. There appeared to be two
manifestations of the condition depending on the age of the bird. In budgerigars
less than 6 months old the feathering was poor with extensive bald or semi-bald
areas on the body and legs, loss of most or all of the large wing and tail
feathers, and loss also of some of the smaller feathers in these sites. The
remaining feathers tended to be of poor quality and sometimes misshapen. Young
affected birds tended to be small for their age. In older birds the contour
feathers were usually unaffected, but all, or some of the primaries, secondaries
and large tail feathers were missing. Regardless of age of the bird the head and
upper neck was spared. In no cases was the beak abnormal. Affected birds seemed
lively and active and no bird died from the condition, although such birds were
kept at the university, only a few months at most.The disease is transmitted to
other birds on the premises both horizontally and vertically, although
transmission tends to be relatively slow. There is no treatment for the disease,
nor are there any preventative treatments in the UK. Measures such as
cleanliness, the testing of new arrivals and the isolation of affected birds,
will reduce the rate of the spread of the disease. An experimental vaccine has
been shown to be effective in the USA. Should fanciers or societies wish to
support further research into this disease I can provide a contact name in
America.
Pulpitis
This is an inflammation of the pulp of
the growing feathers, especially the large ones of the wing and tail. Such
diseased feathers may break off or be shed prematurely. Once shed the feather
may not regrow. A significant proportion of the birds submitted with this
disease had been sent in as "tail-less wonders". Pulpitis is believed to be a
major cause of this condition. The cause of pulpitis is usually a bacterial
infection,predominately Staphylococci or Streptococci although about 15% of
cases are probably of viral origin. These germs are thought to live on the
bird's skin but only cause problems when they invade the growing feathers. There
is a strong correlation between this condition and markedly buff, suggesting
there may be something inherent in buff feathers which makes them susceptible to
this condition. There is also some evidence of vertical transmission.
While in theory, prolonged antibiotic
treatment might cure the condition when a bacteria is the cause, this has not
been tried. Repeated bathing of the birds in Virkon S seems to cure about 40% of
cases. A few appear to recover spontaneously. At the present time there appears
no way of predicting which birds will respond to treatment and which will not.
Mites
Feather mites of a variety of species
are very common on budgerigars, but are not usually seen by the fancier as they
are mostly very small. They are visible only under microscope. They also tend to
live deep in the feathers close to the skin. In the majority of birds they cause
no problems at all. Occasionally mites can cause feather disease in a number of
different ways. There were feather abnormalities in all the cases of mites
listed in the table. Firstly, in heavy mite infestations these active parasites
caused irritation. This in turn led the birds to scratch and bite their plumage,
leading to tatty feathers, or feathers which had been bitten off at various
positions along the shaft. As mites prefer to live on a bird's rump, the tail
feathers are frequently the most affected. Secondly, some species of mite will
eat small segments of the growing feathers, so when they are fully formed a
small part of the feather is seen to be missing. These are most obvious on the
large wing and tail feathers, although the contour feathers can be affected at
times. The third way mites can affect the feathers is by invading the follicles,
which results in the growth of a distorted feather. Only the follicles of the
large feathers of the wing or tail seem big enough for the parasite to enter,
and usually only one or two feathers are affected. These are frequently very
short or horn-like.
The diagnosis of this condition depends
first on finding the mites. However, as these parasites are so common, one has
to eliminate the causes of the feather disease before a diagnosis of the mites
can be made. Treatment with any of the proprietary anti-mite drugs is effective.
Feather
Cysts
It had not been anticipated that this
condition would be such a common cause of feather abnormalities. The other
unexpected feature of this condition was, that in the vast majority of the
cases, the cyst or cysts had not been spotted by the fanciers. The birds had
been sent to me because they had feathers which would not regrow.
Almost all the feather cysts occurred on
the outer parts of the wing or the tail, although some were seen on the necks of
birds. The cysts were of three types, the first and most common for 70% of the
cases, was roughly spherical, up to 1.25cm (just over half an inch) in diameter.
Cysts of this type were either very hard or slightly soft, depending on the
thickness of the fibrous capsule. This capsule surrounded a core of yellow
cheesy material, and the distorted remains of one or more feathers.
The second type was very similar to the
first, but the surrounding skin was inflamed and thickened. As most of the
lesions had not been noticed by the fanciers it was not possible to establish
whether the damage to the skin came before or after the development of the cyst.
The third type was only seen on wings
and accounted for fewer than 10% of cases seen. These were multiple and long and
narrow in shape, lying side by side with one cyst corresponding to the follicle
of one primary feather. They contained cheesy material as in the other cysts but
in a proportion, a very short malformed feather was protruding from the tip of
the cyst.
There was a very strong correlation
between the first two types of cysts and marked buffness. Birds with this type
of inherited plumage have a strong tendency to develop cysts. Unfortunately
fancier's records were insufficiently detailed to show if cysts themselves are
inherited, as is the case with the equivalent condition in canaries (lumps).
Research suggests buff feathering is
linked to feather problems. There is no treatment for this condition other than
surgical removal and it must be borne in mind that the birds will never regrow
the feathers. It is probably not advisable to breed from birds with feather
cysts or from their close relatives.
Stress
Marks
Under this category we will look also at
the absence of barbs and barbules, poorly formed barbs or barbules, and
improperly shed sheaths. Stress marks are lines on the feathers where the
barbules have not formed. Sometimes there is a line of weakness in the shaft at
the same level, and the feather may break off at this point. If the barb and
barbules are absent from a section of the feather, or if they are poorly formed,
the feather does not hold together, giving the bird a tatty appearance.
Improperly shed sheaths is a condition when the feather sheath is not shed in
the usual way and instead persists over a greater part of the feather than is
normal.
Occasionally, fully-formed feathers are
totally enclosed in their sheath. In all three conditions the abnormality is
most easily seen in the large wing and tail feathers.
In all these conditions, the changes
seen in the feathers usually indicate something wrong in the bird's system in
general. This needs to be attended to rather than concentrating on the feathers.
With stress marks, the disturbance is general short lived, a matter of a few
days to a week or thereabouts. The other conditions are associated with longer
periods of illness, which can be quite mild, periods of stress or poor diet.
When one or two birds in a flight are affected, this does not mean that the
overall diet is unsatisfactory. Some budgerigars are very pernickety feeders and
while different foods need to be supplied, it does not mean every bird will eat
all of them. Provided the underlying problem is identified and corrected, the
birds will almost certainly grow a set of normal feathers at the next moult.
Feather
Dusters
This well known condition in which the
feathers of the affected birds grow continually is noted very shortly after the
birds begin to feather up. Such birds usually die at 6 to 8 weeks of age. the
budgerigar that we had for a long time eventually died at 2 years. It grew
contour feathers up to 24cm (9.5 in) long.
Some, and probably all, cases of this
disease have a genetic inherited basis. There is a strong correlation between
affected birds and buff feathering. A paper by Kevin Eatwell in Budgerigar
World said that unaffected siblings of feather dusters are excessively buff
and therefore may be retained for breeding. My view is this is not advisable.
The 8 conditions described above were
responsible for two-thirds of the cases seen. The other 29 diseases accounted
for the remaining third. These will form the second part of this report.
Original text copyright © 1996, Dr John
R Baker
Respiratory Disease in Budgerigars:
an Approach to Treatment
Dr John R Baker
Respiratory disease in
budgerigars is relatively common and, while in some circumstances death is so
rapid that no treatment can be given, in other cases birds can be affected for
long periods and eventually recover. The purpose of this article is to describe
the types of symptoms that may be seen in birds with diseases of the respiratory
system and to detail the steps that owners can take to help to nurse the
affected birds back to health. Reaching the exact diagnosis is sometimes
difficult and a veterinary surgeon experienced in cage bird medicine should
always be consulted before a course of treatment is used. While the use of
terramycin, which most fanciers seem to have, may produce a cure in odd cases,
getting a proper diagnosis and having the proper treatment is much more likely
to result in a cure.
The birds respiratory system
extends from the nostrils (the small holes at the top of the beak) right through
the bird to near the vent. Not only do they have nostrils, a windpipe and lungs
like mammals but in addition, they have a series of structures called air sacs
which sprout out of the lungs. These air sacs are like small balloons which go
around the other internal organs and even extend into the bones of the wing. The
intimate association of the air sacs with the intestines means that diseases of
the intestines may also affect the respiratory system and conversely,
respiratory disease can affect the intestines producing diarrhoea.
There may be a discharge from
the nostrils, this can be either watery or thick and it frequently dries on the
beak and the feathers around the cere. The nostrils may become blocked with this
discharge, either at opening or internally, and occasionally odd objects
breathed in causing blockage; I have seen a budgerigar with a millet seed stuck
up its nose. As in humans, there are a number of sinuses (air spaces opening off
the nose) and these can become affected causing swelling around the head and
this can spread to affect the eyes which become inflamed with a discharge
matting the feathers and sometimes sticking the eyelids together.
Sometimes birds sneeze, and this
again can suggest respiratory disease; they may also cough. Affected birds can
make other noises, there may be wheezing, there may be loss of voice or a change
in the pitch of the song if the bird's voice box is affected. Birds with
breathing difficulties may make clicking noises and this is often associated
with the beak pointing upwards which straightens the trachea and can ease
breathing slightly. If the bird is having difficulty in breathing, the most
obvious sign is tail bobbing. The tail goes up and down in time with the bird's
breathing. If the bird is really having difficulty in getting enough air into
the system, it makes it almost impossible for the bird fly, climb or even perch
and the rocking as it breaths may make it fall off the perch. The bird will
usually feel ill at this stage and may just sit quietly in a corner with its
feathers ruffled up. As death approaches the feet and beak may go blue.
If presented with a bird with
these symptoms what can the owner do as first aid and how can the bird be nursed
when a course of treatment has been started? First of all minimise stress. When
a bird is stressed its need for oxygen increases and if bird is having
difficulty in breathing, added stress may make the difference between life and
death. As in most conditions prompt treatment gives a much better chance of
recovery than will be the case if treatment is delayed, so contact a veterinary
surgeon in your area who has experience in the treatment of cage birds or at
least ask the advice of an experienced fancier. In this context, it should be
stressed that if your vet has prescribed a course of treatment the full course
must be given. The bird may rapidly improve and the temptation is to stop
treatment. If you do this the bird may well relapse and you will be back to
square one again. Affected birds should be isolated from the rest of the stock
as many respiratory diseases are highly infectious and are spread through the
air. Isolation in this case means completely separate from the rest of the birds
in a different room; just putting the diseased bird in a show cage in the bird
room is not isolation. If you have a bird in isolation for
whatever reason, always see to the healthy birds first as in this way you are
less likely to spread the infection about. Where is a temptation to look at the
diseased bird first when you work in the aviary 'just to see how it is', this
temptation must be resisted.
Once a course of treatment has
been started the bird will need nursing care to aid its recovery. First of all
the bird must be kept warm at about 80°F or 27 C. This will not only keep the
bird more comfortable but will reduce the energy requirement and thus the amount
the bird needs to breath. The lighting should be subdued as this appears to
reduce stress and the bird is likely to be more relaxed in semi-darkness. Do not
turn the light off altogether this will stop the bird eating and drinking.
If the bird is having difficulty
breathing, the perches should be lowered, which makes it easier for the bird to
get back on the perch where it will feel better than if it is forced to sit on
the floor. Lowering the perches also means that should the bird fall off it has
less far to drop and is less likely to injure itself. Make sure that food and
water are within easy reach. Discharge from the nostrils or eyes can be cleaned
away with moistened cotton wool and the same technique can be used to open the
eyes if the lids are gummed together. If hard material is seen in the nostrils a
needle can be carefully used to dislodge it.
Many birds that die when they
are diseased, die not of the disease directly, but of either dehydration or
starvation. A useful guide to see if these are taking place is to weigh the bird
at regular intervals and I would suggest that scales to weigh birds would be
worthwhile investment. If weight loss is occurring or if the bird is seen not to
be eating or drinking, the first thing to do is to get some fluid in by crop
tube. Budgerigars have very variable water intakes, but 5ml per day in three or
four doses will be satisfactory. The addition of glucose to the water at two
teaspoonfuls to the pint will supply some energy as well. If the bird has to be
dosed by tube for some time, fruit varieties of baby food, diluted enough to get
it down the tube, are recommended by some authorities and this will provide both
energy and fluid.
The last point that must be
mentioned is that a number of bird respiratory diseases can be caught by humans,
and the most important of these is psittacosis. Should you have sick birds and
you get flu like symptoms with a high temperature, aching joints and a cough, it
is important that you contact the doctor and tell him that you have a sick bird
and that you might have psittacosis. This is important because if caught early
it is easily treated but if left for some time it can become serious and is
occasionally fatal.
Original text Copyright © 1995,
Dr John R Baker
Two Unusual Diseases
Dr John R Baker
During the first year of the diagnostic
service offered to members of the Budgerigar Society and members of the area
societies, common diseases, such as pneumonia and megabacteria, were frequently
seen. However, two conditions were found which I had not seen before, and which
had not been previously reported by other people studying diseases of
Budgerigars or other cage birds. Although rare so far, I will discuss these
briefly as it should be of interest. Both diseases were seen in the birds of one
fancier and both birds also had megabacteria.
Stones
in the Crop
The first case was one of stones, which
had formed in the crop in much the same way that stones can form in humans in
the kidney or gall bladder. The affected budgerigar had two stones in it's crop
They were hard, roughly eggshaped and measured 1.1 and 1.2 centimetres in length
and were off-white in colour. In this particular bird they were causing no
trouble, except that they took up space in the crop so the bird could not eat as
much as normal when feeding. In other birds smaller stones could potentially
move into the lower part of the gullet leading from the crop to the stomach and
become stuck, leading to a rapidly fatal blockage. Stones as large as these
could be felt in the crop, could be seen using an endoscope or diagnosed using
X-rays; the only treatment would be surgical removal. Why the stones formed is
not known.
Internal
Damage
The second case was a bird which was
losing weight and which had diarrhoea. Megabacteria was diagnosed and treated
but the symptoms persisted and the bird eventually died. Post-mortem showed that
the internal damage due to the megabacteriosis was quite severe, but that the
symptoms, at least in part, were due to the presence of an egg in the abdominal
cavity. The egg was collapsed. It was causing a partial obstruction of the
intestines so that the bird's weight loss was partly due to interference with
digestion. The question arises how a fullsized egg, complete with a thin shell,
could get out of the oviduct (where the white and shell are added to the yolk)
into the body cavity.
Two
Possibilities
There appear to be only two possible
routes. First the egg could have passed back up the oviduct and out the top end
where there is an opening, so that the yolk can get into the oviduct from the
ovary. The argument against this is that the oviduct is relatively narrow at the
top, and an egg going back up would be a tight fit. The second possibility is
that the oviduct burst and the egg fell out. As the oviduct is relatively free
of bacteria this would not immediately cause problems. As the bird was not in
laying condition, the oviduct was small and this type of damage could not be
detected. This rare condition could only be diagnosed using X-rays or viewing
the body cavity with an endoscope. The only treatment would be surgical removal
of the egg. As it seems possible that this condition might recur, the oviduct
might also need to be removed to prevent this happening again. Such radical
surgery would render the Budgerigar sterile. Note that this case is different
from so called "internal layers" in which only the yolk goes into the body
cavity. The yolks fall from the ovary but miss the opening at the top end of the
oviduct.
Original text Copyright © 1997, Dr John
R Baker.
Vaccination to control polyomavirus in budgerigars
Branson W. Ritchie, DVM, PhD, Kenneth S. Latimer, DVM, PhD,
Denise Pesti, MS, Raymond Campagnoli, MS, Phil D. Lukert, DVM, PhD
Psittacine Disease Research Group
University of Georgia, College of Veterinary Medicine
Athens, GA 30602
Reprinted in part from Avian Viruses: Function and
Control Wingers Publishing, Lake Worth, FL, 1995.
Budgerigar fledgling disease (BFD), caused by an avian
polyomavirus, was first noted as a clinical syndrome in 1976 and was first
reported as a disease affecting budgerigars in the United States and Canada in
1981.1 Since its initial recognition as an etiologic agent of disease in
psittacine birds, polyomavirus has spread throughout aviaries and the pet trade
to a point where it is a leading cause of death in psittacine birds. 2 While
avian polyomavirus has been shown to be distributed world-wide, there are some
apparent regional differences in the clinical changes associated with infections
in budgerigars. 3-7 1,8 9,10 11 12,13 14-16 For example, in Europe a more
chronic form of the disease is common in budgerigars, while in the United States
and Canada an acute form of disease with high mortality is typical.
Unlike most members of the Papovaviridae family which have
a restricted host range, avian polyomaviruses appear to infect a wide variety of
Psittaciformes (parrots), Passeriformes (weaver finches, canaries) and
gallinaceous birds (chickens and turkeys). 17 Polyomaviruses from budgerigars
have been confirmed to be antigenically related to some virus isolates from
finches and antigenically and genomically related to the polyomavirus isolated
from non budgerigar psittacine birds. 4,18-21 DNA probes have been developed to
detect specific segments of the nucleic acid found in the avian polyomavirus.
These DNA probes, designed from polyomavirus recovered from budgerigars, will
detect the virus in excretions, secretions and infected tissues of non
budgerigar psittacine birds. 6,22,23 The genome of the polyomaviruses isolated
from budgerigars, a blue and gold macaw and a chicken house have been shown to
be closely related. Because of these similarities, it has been suggested that
avian strains of polyomavirus be placed in the subgenus Avipolyomaviurs. 13
The type of clinical disease
induced by polyomavirus in budgerigars seems to be influenced by the age and
condition of a budgerigar when it is exposed to the virus. Budgerigar neonates
from infected flocks may develop normally for 10 to 15 days and then die
suddenly without premonitory signs. 24 Other infected hatchings may develop
clinical signs, including abdominal distention, hemorrhage under the skin and
reduced formation of down and contour feathers. Some infected budgerigars have
been reported to develop neurologic signs characterized by ataxia (incoordination)
and tremors of the head and neck several days before dying. 1,9,24-27 Infected
budgerigars that die shortly after hatching routinely develop more severe and
widespread lesions than do birds in which the morbid state is more prolonged.
The mortality rate associated with naturally acquired avian polyomavirus
infections in young budgerigars may range from 25% to 100%, depending on the age
of the birds. Older birds are considered relatively resistant to disease, while
at the peak of viral activity, up to 100% of exposed budgerigars less than 15
days of age may die. The mortality rate reported for older (greater than 3 weeks
of age) juveniles ranges from 30% to 80%.I,8,9,24-29
Although mortality rates vary with the age of the exposed
birds, polyomavirus infections in young budgerigars usually are rapidly fatal
once clinical signs develop. In most aviary outbreaks, the incidence of
clinically recognized disease will progressively increase during a period of
several months, with peak virus activity occurring during the most active
portion of the breeding season. 25 In one particularly aggressive outbreak of
avian polyomavirus, 90% of young budgerigars were affected within a 2-to 3-week
period.8
Reports vary on the effect of avian polyomavirus on egg
and embryonic development. In one budgerigar aviary, polyomavirus was thought to
have caused an 80% to 90% decrease in egg hatchability at the same time that
hatchling mortality increased from 10% to 60%.8 In another study, there was no
apparent increase in embryonic mortality in eggs from budgerigar hens that were
considered persistently infected with avian polyomavirus. Additionally, many of
the chicks from these hens were found to be infected, but remained asymptomatic.
30 Findings from the first report would suggest that avian polyomavirus can
cause embryonic deaths and decreased hatchability, while findings from the
second study would suggest that avian polyomavirus infections do not cause
problems in embryonic development. It is possible that both findings are correct
and the effect of the virus may depend on as yet unidentified factors. Like
mammals, budgerigars that recover from polyomavirus infections are thought to
remain persistently infected.30-32 Evidence suggests that these persistently
infected budgerigars are responsible for the spread of this virus through
budgerigar flocks and within the avicultural industry.
1,21,31,14 Stress associated with changes in weather,
diet, breeding or concomitant disease may cause persistently infected
budgerigars to shed virus resulting in outbreaks of disease. In polyomavirus
outbreaks at 23 different budgerigar aviaries, the onset of disease could be
traced to the addition of new, clinically normal breeders. 1,25
Polyomavirus inclusion bodies frequently can be detected
in feathers, feather follicles, spleen, liver and Kidney from persistently
infected, clinically normal adult budgerigars. 25,26 Viral inclusion bodies are
common in the renal tubular epithelium. Persistence of virus in the kidneys of
budgerigars, with subsequent excretion in the urine, has been proposed as a
method of virus transmission. 1,26,29,33,35 Viral nucleic acid has been detected
in the excrement, feathers and oral mucosa of clinically normal budgerigars. 36
The incidence of polyomavirus activity in budgerigars is
high. In one study, virus was demonstrated by histology and virus isolation from
all of 10 budgerigars with clinical signs of avian polyomavirus and from 5 of 10
(50%) clinically healthy budgerigars. 26 In another study, 4 of 7 (57%) nestling
budgerigars had microscopic changes suggestive of avian polyomavirus, while all
of 7 nestlings had viral nucleic acid in their tissues. 30 Polyomavirus nucleic
acid was demonstrated in the serum of 9 of 12 (75%) 9-to 13-day-old budgerigars
from a flock in which virus activity was considered enzootic. 34 The possibility
that up to, 100% of the budgerigars in some flocks could be infected with avian
polyomavirus was first discussed in 1984.25 This has been confirmed in
subsequent studies using viral-specific DNA probes. In one study involving a
flock of budgerigars with a history of repeated polyomavirus-induced disease,
all of 40 clinically normal budgerigars had polyomavirus nucleic acid in their
tissues. Virus-neutralizing antibodies to avian polyomavirus were detected in
all 144 of another group of budgerigars. 30 Experimental data and observations
with the natural disease suggest that polyomavirus transmission in budgerigars
may occur by both horizontal and vertical routes, that is, both among members of
a flock and between generations from parent to offspring. 1,5,27,28,30
Experimentally infected 3-to 10-day-old budgerigar neonates died 11 days after
being given BFD virus intramuscularly. 37 When 25-day-old budgerigars were
exposed intranasally to virus collected from the skin of diseased birds, they
developed microscopic lesions characteristic of a polyomavirus infection but
remained clinically normal. 1,25 These findings suggest that inhalation of
aerosolized virus could be a natural route through which polyomavirus enters a
susceptible bird. In one trial, young seronegative budgerigars seroconverted
within 16 days after being placed in the same enclosure with seropositive birds.
Seronegative budgerigars also seroconverted when they were
placed in enclosures adjacent to those containing seropositive birds. These
finding suggest that direct and indirect transmission of polyomavirus can occur.
27
Unvaccinated susceptible psittacine birds may be exposed
to polyomavirus through exposure to virus-contaminated feces, feather dust,
urates and respiratory secretions. 17,25 Until polyomavirus can be controlled in
budgerigars through a widespread vaccination program, it should be considered
dangerous to house budgerigars in the same air space with unvaccinated non
budgerigar psittacine birds. The potential for intraspecies transmission of
polyomavirus may be a particular problem for pet retailers that maintain both
budgerigars and non budgerigar psittacine birds. In one environmental study, it
was found that polyomavirus nucleic acid could be detected using DNA
amplification and detection techniques in all of 5 samples collected from 3
different pet shops which housed budgerigars. (Dr. Nancy Jaax, personal
communication) Budgerigars should be considered the primary reservoir of
polyomavirus. 'The potential for budgerigars to continue to spread this
environmentally stable virus among unvaccinated psittacine birds is cause for
action.
Controlling Polyomavirus in Budgerigars
It has been suggested that polyomavirus disease-free
budgerigar nestlings can be produced by interrupting the breeding cycle,
removing all but the older breeding birds and disinfecting the aviary. 8
Breeding cycle manipulation was used to control polyomavirus infections in
budgerigars, after hatchability of fertile eggs dropped from 80% to 90% at the
same time that hatching mortality increased from 10% to 60% in an aviary
experiencing an avian polyomavirus outbreak. By interrupting the breeding cycle
for 7 months and thoroughly cleaning the aviary with bleach, return to 80%
hatchability and a cessation of hatching deaths was achieved when breeding
resumed. However, in other affected aviaries, mortalities and disease have
continued when breeding was resumed following a rest period. 8 In another study,
it was demonstrated that older breeding budgerigars shed fewer virus particles
and less frequently than young adults. 30,32 Any attempt to manage polyomavirus
through manipulation of the breeding cycle has the inherent problem that
persistently infected budgerigars, and thus a future source for virus shedding,
remain in the flock.
In budgerigars, which are known to develop persistent
polyomavirus infections, the detection of polyomavirus-neutralizing antibodies
can be used to screen for infected budgerigars which should be considered a risk
for transmitting the virus. 34,38 Testing budgerigars for the presence of
virus-neutralizing antibodies and culling positive birds has been suggested as a
method to establish flocks of polyomavirus-free budgerigars. 33,36 Additionally,
depopulation of budgerigar aviaries experiencing outbreaks, followed by
restocking with seronegative birds has been suggested as a method of controlling
enzootic infections in this species. 8,33,36 The practical problem with using
this testing strategy for controlling polyomavirus is that once a seronegative
flock of budgerigars has been established, the flock would then be highly
suceptible (theoretically 100% susceptible) to polyomavirus infection if the
virus were inadvertently introduced to the aviary. However, by using a serologic
assay to establish "polyomavirus-free" budgerigars followed by vaccination to
protect the budgerigars from subsequent infection, it should be possible to
prevent polyomavirus-induced disease in the budgerigar aviary and reduce the
role that budgerigars play as a reservoir for polyomavirus in the pet trade.
In non budgerigar psittacine birds, polyomavirus can be
controlled using an inactivated avian polyomavirus vaccine. 3944 Controlling
polyomavirus infections in budgerigars presents a different set of problems. A
budgerigar infected with polyomavirus is considered to be infected for life and
can shed the virus for long periods of time. This high prevalence of
polyomavirus in budgerigars, and the fact that an infected budgerigar continues
to shed for extended periods, renders vaccination alone of questionable value in
controlling the virus in a budgerigar flock. Additionally, the cost of the
inactivated vaccine makes it difficult to economically justify in commercial
budgerigar flocks. None-the-less, it is important for the avicultural industry
to control polyomavirus in budgerigars because this group of birds is serving as
a reservoir for the virus. For the past year, we have been evaluating an
economically feasible testing and vaccination program to control polyomavirus in
budgerigars. We are currently testing this program in flocks of budgerigars and
our data is encouraging.
This vaccine* was developed by identifying a virus isolate
which, when administered intramuscularly, will cause an immune response without
inducing virus shedding or detectable persistence of the virus. In one safety
and immunogenicity trial, 4 recently weaned budgerigars with no detectable
polyomavirus-neutralizing antibodies were injected intramuscularly with the
experimental vaccine. Another budgerigar that was housed in the same enclosure
with these vaccinates served as a contact control. None of the vaccinates had
any clinically recognizable adverse reactions following vaccination.
The experimental vaccine designed for use in budgerigars
was found to stimulate an immune response in birds inoculated intramuscularly
but not in birds inoculated by the combined intraoral, intranasal and
intraocular routes. A group of 5 recently weaned budgerigars that were negative
for polyomavirus-neutralizing antibodies was used in this safety and
immunogenicity trial. Two birds (#6 and #7) were inoculated by the combined
intraoral, intranasal and intraocular routes with experimental vaccine. Three
birds (#8, #9 and #10) were injected with the same suspension intramuscularly.
Serum was collected from each bird on day 0, 10 and 20. None of the vaccinates
had any clinically recognizable adverse reactions.
The results of this study indicated that the experimental
budgerigar polyomavirus vaccine induced a detectable virus-neutralizing antibody
response when injected intramuscularly (considered an unnatural route of
exposure) but not in the birds inoculated by the combined intraoral, intranasal
and intraocular routes (considered a natural route of exposure). This finding
suggests that the polyomavirus contained in the experimental vaccine has been
sufficiently altered to prevent it from inducing an infection when a bird is
exposed by a natural route. In an additional safety and immunogenicity trail, 7
mature budgerigars were inoculated intramuscularly with the experimental
budgerigar polyomavirus vaccine. Two birds were housed in the same enclosure
with the vaccinates and served as contact controls. Serum was tested for
virus-neutralizing antibodies on day 0 (which was also the day of vaccination)
and approximately 1 month later. None of the vaccinates had any clinically
recognizable adverse reactions.
These safety and immunogenicity trials suggest that the
experimental budgerigar vaccine will induce an immune response, as detected by a
significant increase in virus-neutralizing antibodies, when injected
intramuscularly and that vaccinates do not shed a sufficient quantity of viable
virus to be a risk for infecting birds with which they are in direct contact. It
is anticipated that the ongoing studies which are necessary to obtain USDA
registration of this vaccine will further confirm its safety and immunogenicity
in budgerigars.
Vitamin and Mineral Supplement
Poisoning
Dr John R. Baker
The
Liverpool University Budgerigar Ailment Research
Project, sponsored by the Lancashire, Cheshire and North Wales
including the Isle of Man Budgerigar Society, was started in 1984 and ran for
8 years.
I had noticed when talking to fanciers
that many used a great variety of mineral and vitamin supplements and it was not
unusual for 2, 3 or even more of these to be used in one stud. This led to
concern that there was the distinct possibility of birds being poisoned; indeed
vitamin A poisoning was diagnosed in a stud of birds at about this time.
It was decided to investigate this in
1989-90. The work which was done indicated that vitamin A poisoning was a
distinct possibility if certain of the commercial supplements were mixed. On the
other hand vitamin D3 poisoning, while it was produced experimentally, was
unlikely to occur in fanciers' birds.
This work also indicated the minimum
levels of these vitamins and from this point of view all the commercial
available supplements were satisfactory. The moral of this work was that
fanciers should use only one of these supplements and that the manufacturer's
instruction should be followed.
Original text Copyright © 1990, Dr John
R Baker.
Vomiting
Dr John R. Baker
The
Liverpool University Budgerigar Ailment Research
Project, sponsored by the Lancashire, Cheshire and North Wales
including the Isle of Man Budgerigar Society, was started in 1984 and ran for
8 years.
1985-6 was the year of vomiting budgies.
Birds actually being sick or or trying to vomit were noticed by a number of
fanciers as being a common problem. As with 'going light' the fancy came up
trumps and provided large numbers of birds for post-mortem investigation. This
showed that affected birds had a disease of the oesophagus (gullet) and crop
which were inflamed and partly obstructed and that this was producing the
symptoms.
After a number of false leads this
disease was found to be due to a minute parasite called Trichomonas gallinae.
A number of treatments were already available for this condition in pigeons and
one of these in particular (Emtryl soluble) was adapted to budgerigars and was
shown to be highly effective in curing this condition and eliminating the
disease from studs.
The only major problem was that some
fanciers would just not believe that the minute amount of the drug needed was
enough and as a result they massively overdosed. More than one fancier almost
wiped out their entire stud due to the toxic effects of very large doses. The
moral of this is always use in accordance with the vet's instructions or follow
the manufacturer's information to the letter.
Original text Copyright © 1986, Dr John
R Baker

| 



